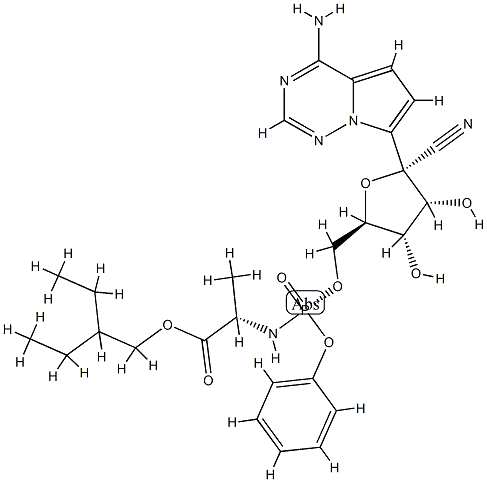
Remdesivir synthesis
- Product Name:Remdesivir
- CAS Number:1809249-37-3
- Molecular formula:C27H35N6O8P
- Molecular Weight:602.58
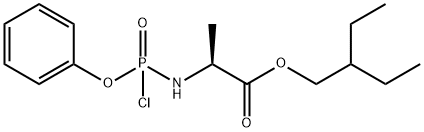
1355049-92-1
18 suppliers
inquiry
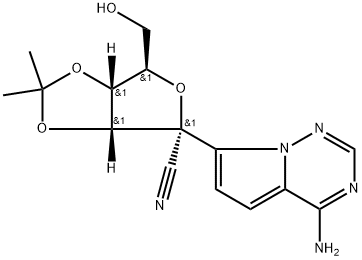
1191237-80-5
108 suppliers
inquiry

1809249-37-3
305 suppliers
$96.00/5mg
Yield:1809249-37-3 73%
Reaction Conditions:
Stage #1: (3aR,4R,6R,6aR)-4-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-6-(hydroxymethyl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxole-4-carbonitrilewith 2,6-dimethylpyridine;C17H21N3O2 in dichloromethane at -20 - 20; for 0.333333 h;Molecular sieve;Inert atmosphere;
Stage #2: 2-ethylbutyl (2S)-2-{[chloro(phenoxy)phosphoryl]amino}propanoate in dichloromethane at -20; for 24 h;
Stage #3: with toluene-4-sulfonic acid in methanol at 20; for 24 h;
Steps:
2
In a flame dried round bottom flask, a mixture of acetonide-nucleoside acceptor 7 (1.0 g, 3.02 mmol) and catalyst 29 (180 mg, 0.62 mmol) were co-evaporated with anhydrous toluene (20 mL, 2 times), dried for 2 h on high-vacuum. The mixture was dissolved in dry CH2Cl2 (50 mL) and then freshly activated 4? molecular sieves (1.5 g) followed by 2,6-luditine (700 μL, 6.04 mmol) were added. The suspension was then stirred under N2 atmosphere at room temparature for 10 min, and then cooled to -20 °C. After 10 min, a solution of phosphochloridate 4 (1.57 g, 4.53 mmol) in dry CH2Cl2 (5 mL) was added and the reaction mixture was allowed to stir at same temperature for 24 h. TLC analysis indicates the complete conversion of starting materials into the products. Then the CH2Cl2 was evaporated by vacuum. Later on, the crude material was treated with 10.0 equiv of p-TSA in MeOH (50 mL). The reaction mixture was allowed to stir for 24 h at room temperature and the TLC analysis indicated the product formation. Then the reaction mixture was filtered through a pad of Celite to remove 4? molecular sieves, quenched by adding Et3N and concentrated in vacuo. The crude residue was dissolved in EtOAc and washed with Saturated NaHCO3, the aqueous layer was back extracted with EtOAc (3 x 50 mL) and the combined organic layers were dried over MgSO4, filtered and concentrated in vacuo. The crude residue was subjected to silica gel column chromatography by using EtOAc in hexane (0→ 100%) as an eluent to afford the products as a foamy solid. The diastereomeric mixture was purified by using C 18 column to give compound 1 as a foamy solid (1.39 g, 73%). 1H NMR (600 MHz, CD3OD) δ 7.87 (s, 1H), 7.30 (t, J = 7.8 Hz, 2H), 7.21- 7.14 (m, 3H), 6.91 (d, J= 4.6 Hz, 1H), 6.88 (d, J= 4.6 Hz, 1H), 4.79 (d, J= 5.4 Hz, 1H), 4.42-4.35 (m, 2H), 4.28 (dt, J= 10.5, 5.1 Hz, 1H), 4.17 (t, J= 5.6 Hz, 1H), 4.02 (dd, J= (0148) 10.9, 5.8 Hz, 1H), 3.90 (ddd, J= 23.7, 12.7, 6.4 Hz, 2H), 1.45 (dt, J= 12.3, 6.2 Hz, 1H), 1.34-1.28 (m, TH), 0.85 (t, J= 7.5 Hz, 6H). 13C NMR (150 MHz, CD3OD) δ 175.15, 175.1, 157.4, 152.3, 152.3, 148.4, (0150) 130.9, 126.2, 125.7, 121.51, 121.5, 118.1, 117.7, 112.5, 102.8, 84.44, 81.4, 84.4, 75.9, 71.8, 68.2, 67.3, 67.27, 41.8, 24.4, 24.3, 20.7, 20.6, 11.5, 11.4.
References:
WO2022/76638,2022,A1 Location in patent:Paragraph 0137; 0139-0142