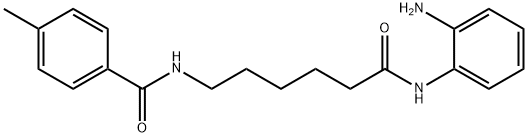
RGFP 109 synthesis
- Product Name:RGFP 109
- CAS Number:1215493-56-3
- Molecular formula:C20H25N3O2
- Molecular Weight:339.43
Yield: 33.9%
Reaction Conditions:
with benzotriazol-1-ol;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride;triethylamine in dichloromethane at 0;Inert atmosphere;
Steps:
7
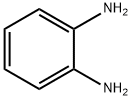
N-[6-(2-aminophenylamino)-6-oxohexyl]-4-methylbenzamide (R01) A mixture of 6-(4-methylbenzamido)hexanoic acid (498 mg, 2 mmol), o-phenylenediamine (216 mg, 2 mmol), EDCI (383 mg, 2 mmol), HOBt (405 mg, 3 mmol), and triethylamine (404 mg, 4 mmol) in dichloromethane (30 mL) was stirred at room temperature under nitrogen overnight. The reaction mixture was washed with water and brine, dried over Na2SO4, and evaporated. The residue was purified by chromatography on silica gel to give the title compound (227 mg, 33.9%) as a white solid. 1H NMR (DMSO): δ 9.06 (s, 1H), 8.35 (s, 1H), 7.73 (d, J=3.0 Hz, 2H), 7.24 (d, J=3.0 Hz, 2H), 7.14 (1H, J=3.0, d), 6.86-6.89 (m, 1H), 6.70 (d, J=3.0 Hz, 1H), 6.50 (M, 1H), 4.80 (s, 2H), 3.22-3.26 (m, 2H), 2.30-2.35 (m, 5H), 1.53-1.65 (m, 4H), 1.36-1.38 (m, 2H). LC-MS: 340 (MH)+. purity>95%.
References:
REPLIGEN CORPORATION US2012/94971, 2012, A1 Location in patent:Page/Page column 40
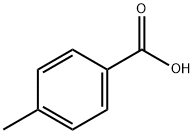
99-94-5
569 suppliers
$9.00/1g

1215493-56-3
87 suppliers
$49.00/5mg
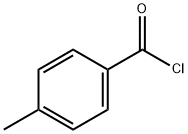
874-60-2
397 suppliers
$10.00/5g

1215493-56-3
87 suppliers
$49.00/5mg
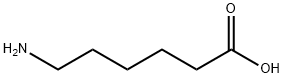
60-32-2
590 suppliers
$5.00/10g

1215493-56-3
87 suppliers
$49.00/5mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1215493-56-3
87 suppliers
$49.00/5mg
![Hexanoic acid, 6-[(4-Methylbenzoyl)aMino]-](/CAS/20150408/GIF/78521-43-4.gif)