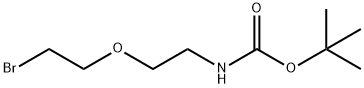
Br-PEG1-NHBoc synthesis
- Product Name:Br-PEG1-NHBoc
- CAS Number:164332-88-1
- Molecular formula:C9H18BrNO3
- Molecular Weight:268.15
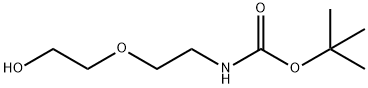
Alcohol 64 (1 .25 g, 6.09 mmol) was dissolved in DCM (34 mL). The solution was cooled to 0°C and methanesulfonyl chloride (MsCI, 0.80 mL, 10.3 mmol, 1 .7 equiv) was added to the solution followed by Et3N (1.9 mL, 13.4 mmol, 2.2 equiv). After stirring for 3 h at rt, the reaction mixture was diluted with acetone (33 mL) and LiBr (8.9 g, 103 mmol, 17 equiv) was added. The reaction mixture was stirred overnight at rt. After that time, the solvents were evaporated under reduced pressure. The crude residue was diluted with EtOAc and washed with H20 and brine. The organic phase was dried over anhyd Na2S04. The suspension was filtered over cotton and the filtrate concentrated in vacuo. Purification by flash chromatography eluting with PE/Acetone (85:15→ 8:2) gave bromide 65 (1 .60 g, 5.95 mmol, 98%) as a colourless oil.
139115-91-6
130 suppliers
$5.00/1g

164332-88-1
83 suppliers
inquiry
Yield: 98%
Reaction Conditions:
Stage #1:2-(2-tert-butyloxycarbonylaminoethoxy)ethanol with methanesulfonyl chloride;triethylamine in dichloromethane at 0 - 20; for 3 h;
Stage #2: with lithium bromide in dichloromethane;acetone at 20;
Steps:
tert-butyl (2-(2-bromoethoxy)ethyl)carbamate (65)
Alcohol 64 (1 .25 g, 6.09 mmol) was dissolved in DCM (34 mL). The solution was cooled to 0°C and methanesulfonyl chloride (MsCI, 0.80 mL, 10.3 mmol, 1 .7 equiv) was added to the solution followed by Et3N (1.9 mL, 13.4 mmol, 2.2 equiv). After stirring for 3 h at rt, the reaction mixture was diluted with acetone (33 mL) and LiBr (8.9 g, 103 mmol, 17 equiv) was added. The reaction mixture was stirred overnight at rt. After that time, the solvents were evaporated under reduced pressure. The crude residue was diluted with EtOAc and washed with H20 and brine. The organic phase was dried over anhyd Na2S04. The suspension was filtered over cotton and the filtrate concentrated in vacuo. Purification by flash chromatography eluting with PE/Acetone (85:15→ 8:2) gave bromide 65 (1 .60 g, 5.95 mmol, 98%) as a colourless oil. 1H-NMR (500 MHz, CDCI3): δ 4.91 (s, 1 H), 3.78 (t, 2H), 3.56 (t, 2H), 3.47 (t, 2H), 3.33 (d, 2H), 1 .45 (s, 9H).
References:
UNIVERSITÄT BASEL;HERRENDORFF, Ruben;ERNST, Beat;STECK, Andreas;PFISTER, Hélène;NAVARRA, Giulio WO2017/46172, 2017, A1 Location in patent:Page/Page column 83; 84
24424-99-5
817 suppliers
$13.50/25G

164332-88-1
83 suppliers
inquiry

![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)