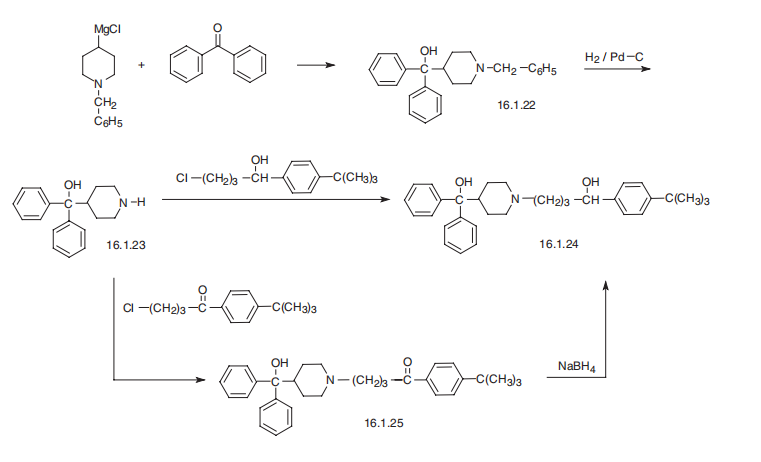
Terfenadine synthesis
- Product Name:Terfenadine
- CAS Number:50679-08-8
- Molecular formula:C32H41NO2
- Molecular Weight:471.67
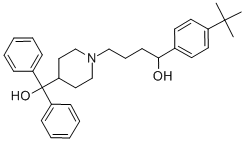
![4'-tert-butyl-4-[4-(hydroxybenzhydryl)piperidino]butyrophenone](/CAS/GIF/43076-30-8.gif)
43076-30-8
13 suppliers
$180.00/100mg

50679-08-8
198 suppliers
$25.00/500mg
Yield:50679-08-8 76%
Reaction Conditions:
with methanol;sodium tetrahydroborate at 20; for 3 h;
Steps:
II 1-(4-(tert-butyl)phenyl)-4-(4-(hydroxydiphenylmethyl)piperidin-1-yl)butan-1-ol
1-(4-(tert-butyl)phenyl)-4-(4-(hydroxydiphenylmethyl)piperidin-1-yl)butan-1-ol To a vial was added the 1-(4-(tert-butyl)phenyl)-4-(4-(hydroxydiphenylmethyl)piperidin-1-yl)butan-1-one (KSC-335-007 (0.198 g, 0.422 mmol) and MeOH (2 mL). The sodium borohydride (0.032 g, 0.844 mmol) was then added and the reaction stirred at rt for 3 h. The reaction was concentrated to dryness, water (5 mL) was added and a white precipitate formed. The precipitate was filtered out and then dissolved in DCM (10 mL), dried with MgSO4, filtered and concentrated to produce pure 1-(4-(tert-butyl)phenyl)-4-(4-(hydroxydiphenylmethyl)piperidin-1-yl)butan-1-ol (0.151 g, 0.320 mmol, 76% yield) as on oil. 1H NMR (400 MHz, CDCl3): δ 7.52-7.46 (m, 4H), 7.33-7.25 (m, 8H), 7.21-7.15 (m, 2H), 4.61-4.56 (m, 1H), 3.16-3.11 (br m, 1H), 3.00-2.94 (m, 1H), 2.51-2.34 (m, 4H), 2.10-1.88 (m, 4H), 1.83-1.75 (m, 1H), 1.70-1.45 (m, 6H), 1.30 (s, 9H). 13C NMR (125 MHz, CDCl3): δ 149.4, 146.1, 146.0, 142.7, 128.2, 128.1, 126.4, 126.3, 125.7, 125.6, 125.3, 125.0, 79.2, 73.4, 58.9, 54.7, 53.3, 44.2, 39.7, 34.4, 31.4, 26.0, 25.9, 24.1. LCMS Retention time: 4.137 min. LCMS purity 97.5%. HRMS (ESI): m/z calcd for C32H41NO2 [M+H]+ 472.3144. found 472.3219.
References:
UNIVERSITY OF ROCHESTER;UNIVERSITY OF KANSAS;Dunman, Paul M.;Krysan, Damian J.;Flaherty, Daniel P. US2015/238473, 2015, A1 Location in patent:Paragraph 0210

863029-85-0
1 suppliers
inquiry
201230-82-2
1 suppliers
inquiry

115-46-8
400 suppliers
$10.00/1g

50679-08-8
198 suppliers
$25.00/500mg

115-46-8
400 suppliers
$10.00/1g

50679-08-8
198 suppliers
$25.00/500mg

43076-61-5
215 suppliers
$71.00/25g

50679-08-8
198 suppliers
$25.00/500mg