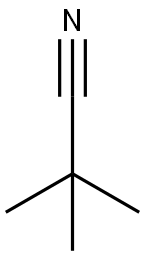
Trimethylacetonitrile synthesis
- Product Name:Trimethylacetonitrile
- CAS Number:630-18-2
- Molecular formula:C5H9N
- Molecular Weight:83.13
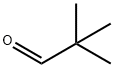
630-19-3
299 suppliers
$13.00/1g

630-18-2
173 suppliers
$10.00/1g
Yield:630-18-2 64 %Chromat.
Reaction Conditions:
with ammonium acetate;acetic acid;4-acetylamino-2,2,6,6-tetramethylpiperidine-1-oxoammonium tetrafluoroborate at 70; for 12 h;Inert atmosphere;
Steps:
General procedures for 4-AcNH-TEMPO+BF4- mediated nitriles synthesis
General procedure: A 15 mm flame-dried test tube, which was equipped with a magnetic stir bar and charged with aldehyde (0.3 mmol, in case of solid), 4-AcNH-TEMPO+BF4- (2.0 equiv, 0.6 mmol), and NH4OAc (4.0 equiv, 1.2 mmol), was evacuated and backfilled with nitrogen (this process was repeated 3 times). After 0.3 mL of AcOH was added, aldehyde (0.3 mmol, in case of liquid), and AcOH (0.3 mL) were added in sequence. The reaction mixture was stirred for 12 h at 70 oC under N2 balloon, and then cooled to room temperature. The reaction was diluted by adding EtOAc and washed 4 M HCl aqueous solution. Two layers were separated, and the aqueous layer was extracted with EtOAc. The combined organic layers were washed with Na2CO3 aqueous solution. The organic layer was dried over MgSO4, filtered, and concentrated to a volume of approximately 20 mL by evaporator. To eliminate remaining aldehyde, aqueous 2 M Na2S2O5 aqueous solution (20 mL) was added to the organic layer and stirred for 2 hours. Two layers were separated, and the organic layer was dried over MgSO4, filtered, and concentrated in vacuo. The residue was purified by column chromatography to give nitrile products.
References:
Kim, Myeong Jin;Mun, Junyoung;Kim, Jinho [Tetrahedron Letters,2017,vol. 58,# 50,p. 4695 - 4698] Location in patent:supporting information
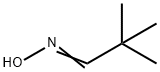
637-91-2
21 suppliers
$45.00/10mg

630-18-2
173 suppliers
$10.00/1g
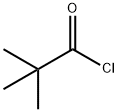
3282-30-2
356 suppliers
$18.00/100ml

630-18-2
173 suppliers
$10.00/1g
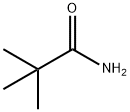
754-10-9
169 suppliers
$7.00/5g

630-18-2
173 suppliers
$10.00/1g

143-33-9
5 suppliers
$10.00/1g
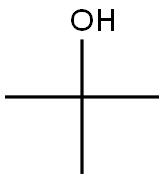
75-65-0
737 suppliers
$10.00/10ml

630-18-2
173 suppliers
$10.00/1g

762-84-5
36 suppliers
$60.39/10g