| Identification | Back Directory | [Name]
Palonosetron | [CAS]
135729-61-2 | [Synonyms]
2-Qhbiqo
Rs 25233-197
PALONOSETRON
Palonosetron
Palonosetron [inn]
Palonosetron hydrochloride salt
(S)-2-((S)-quinuclidin-3-yl)-2,3,3a,4,5,6-hexahydro-1H-benzo[de]isoquinolin-1-one
2-(1-Azabicyclo(2.2.2)oct-3-yl)-2,3,3A,4,5,6-hexahydro-1H-benz(de)isoquinolin-1-one
(S-(R*,R*))-2-(1-Azabicyclo(2.2.2)oct-3-yl)-2,3,3A,4,5,6-hexahydro-1H-benz(de)isoquinolin-1-one
1H-Benz[de]isoquinolin-1-one, 2-(3S)-1-azabicyclo[2.2.2]oct-3-yl-2,3,3a,4,5,6-hexahydro-, (3aS)-
1H-Benz(de)isoquinolin-1-one, 2-(1-azabicyclo(2.2.2)oct-3-yl)-2,3,3A,4,5,6-hexahydro-, (S-(R*,R*))- | [EINECS(EC#)]
1312995-182-4 | [Molecular Formula]
C19H24N2O | [MOL File]
135729-61-2.mol | [Molecular Weight]
296.41 |
| Chemical Properties | Back Directory | [Melting point ]
87-88° | [alpha ]
D -136° (c = 0.25 in chloroform) | [Boiling point ]
470.4±45.0 °C(Predicted) | [density ]
1.24±0.1 g/cm3(Predicted) | [storage temp. ]
Store at -20°C | [solubility ]
DMSO | [form ]
Powder | [pka]
9.77±0.33(Predicted) |
| Hazard Information | Back Directory | [Description]
This selective and conformationally restricted 5-HT3
receptor antagonist was approved for the treatment of
chemotherapy-induced nausea and vomiting. The drug
was originally developed by Syntex Corp (now Roche
Bioscience) and is currently being developed by Helsinn and
MGI Pharm. | [Uses]
Palonosetron is a serotonin 5-HT3 receptor antagonist used in the prevention and treatment of chemotherapy-induced nausea and vomiting (CINV). Antiemetic. | [Definition]
ChEBI: An organic heterotricyclic compound that is an antiemetic used (as its hydrochloride salt) in combination with netupitant (under the trade name Akynzeo) to treat nausea and vomiting in patients undergoing cancer chemotherapy. | [Clinical Use]
Anti-emetic:
For use with cancer chemotherapy | [Synthesis]
(S)-3-Aminoquinuclidine was condensed with
inexpensive 1,8-naphthalic anhydride (138) to furnish imide
139 in 93% yield and isolated as its TFA salt. Imide
139 was hydrogenated at 5 psi to give intermediate 140 with
one of the reduced aromatic ring. The less hindered C-3
carbonyl group in 140 was selectively reduced to a hydroxy
group by using sodium borohydride in ethanol under
nitrogen at low temperature to give intermediate 141.
Intermediate 141 was not isolated because of the formation
of a tight boron complex. Subsequently, acid was added to
141 in i-PrOH to decompose the boron complex and
dehydrate intermediate 141 to 142, which was conveniently isolated as its HCl salt in 75% yield from 139. Palonosetron
(XVIII) was obtained in 57% yield by palladium-catalyzed
hydrogenation of 142.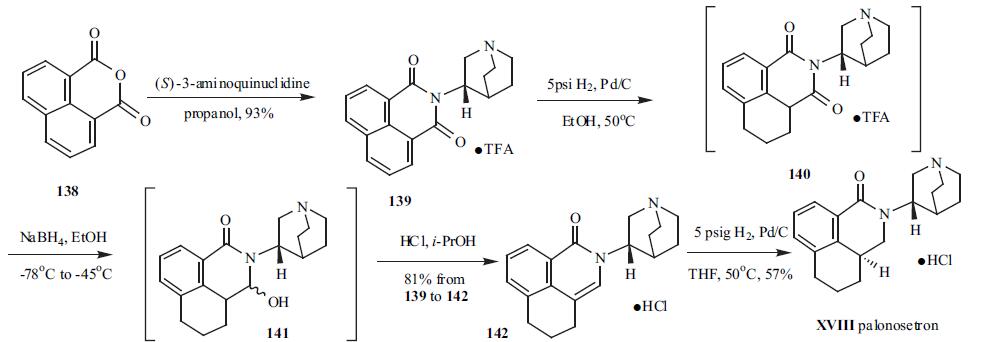
| [Enzyme inhibitor]
This serotonin 5-HT3 receptor antagonist and longlasting anti-emetic (FW = 296.41 g/mol; CAS 135729-61-2), known by code name RS 25259, RS 25259-197, trade name Aloxi?, and systematic name (3aS)-2-[(3S)-1- azabicyclo[2.2.2]oct-3-yl]-2,3,3a,4,5,6-hexahydro-1H-benz[de]isoquinolin- 1-one, is used to treat chemotherapy-induced nausea/vomiting. Polonosetron affects both peripheral and central nervous systems, reducing vagus nerve activity, thereby deactivating the 5-HT3 receptor-dense vomiting center located in the medulla oblongata. It is without effect on dopamine receptors or muscarinic receptors. Primary Mode of Inhibitor Action: Intravenously administered palonosetron has a linear pharmacokinetic profile, with a long terminal elimination half-life of ~40 hours and moderate (62%) plasma protein binding. Its high affinity and long half-life most likely explains its persistent antiemetic effect. Similar 5- HT3 antagonists include tropisetron, ondansetron, dolasetron, and granisetron. The effectiveness of each depends on particular variants of 5- HT3 receptors expressed by the patient, including changes in promoters for the receptor genes | [Drug interactions]
Potentially hazardous interactions with other drugs
None known | [Metabolism]
Palonosetron is eliminated by a dual route, about 40%
eliminated through the kidney and approximately
50% metabolised by CYP2D6, and to a lesser extent,
CYP3A4 and CYP1A2 isoenzymes in the liver to form
two primary metabolites, which have less than 1% of
the 5HT3
receptor antagonist activity of palonosetron.
After a single intravenous dose of [14C]-palonosetron,
approximately 80% of the dose was recovered within
144 hours in the urine with unchanged palonosetron
representing approximately 40% of the administered dose. | [storage]
Store at -20°C |
|
|