| Identification | Back Directory | [Name]
Imidafenacin | [CAS]
170105-16-5 | [Synonyms]
Uritos
Staybla
ONO 8025
4-(2-methyL
Imidafenacin
Midana new API
)-2,2-diphenyL
Imidafenacin-d10
KRP-197; ONO-8025
IMidafenacin DISCONTINUED-PATENTED PRODUCT
2-Methyl-α,α-diphenyl-1H-iMidazole-1-butanaMide
4-(2-methylimidazol-1-yl)-2,2-diphenylbutanamide
1H-IMidazole-1-butanaMide,2-Methyl-a,a-diphenyl-
1H-Imidazole-1-butanamide, 2-methyl-α,α-diphenyl-
4-(2-Methyl-1-iMidazolyl)-2,2-
diphenylbutanaMide
4-(2-METHYL-1H-IMIDAZOL-1-YL)-2,2-DIPHENYLBUTANAMIDE | [EINECS(EC#)]
689-703-7 | [Molecular Formula]
C20H21N3O | [MDL Number]
MFCD09833703 | [MOL File]
170105-16-5.mol | [Molecular Weight]
319.4 |
| Chemical Properties | Back Directory | [Melting point ]
184-187°C | [Boiling point ]
579.7±50.0 °C(Predicted) | [density ]
1.12±0.1 g/cm3(Predicted) | [storage temp. ]
Sealed in dry,Room Temperature | [solubility ]
DMSO, Methanol | [form ]
Solid | [pka]
15.72±0.50(Predicted) | [color ]
White |
| Hazard Information | Back Directory | [Description]
Imidafenacin, an M3/M1 muscarinic receptor antagonist, was introduced in
Japan for the oral treatment of OAB. The majority of OAB symptoms are
thought to result from overactivity of the detrusor muscle, which is primarily
mediated by acetylcholine-induced stimulation of muscarinic M3 receptors in the
bladder. Previously marketed muscarinic antagonists for OAB include propiverine,
tolterodine, oxybutynin, trospium, darifenacin, and solifenacin. In vitro,
imidafenacin is equally active against M1 and M3 receptors (Kb=0.32 and
0.55nM, respectively), and approximately 10-fold less active against M2 receptors
(Kb=4.13nM).
Imidafenacin is chemically synthesized in three steps starting with alkylation of diphenylacetonitrile with dibromoethane, followed by condensation with 2-methylimidazole, and hydrolysis of the cyano group to a carboxamide group with 70% sulfuric acid. | [Chemical Properties]
White Solid | [Originator]
Kyorin (Japan) | [Uses]
A novel therapeutic agent for overactive bladder with antimuscarinic activity, on mediator release from urothelium and detrusor overactivity induced by cerebral infarction. A muscarinic antagonist. | [Definition]
ChEBI: Imidafenacin is a diarylmethane. | [Brand name]
Staybla | [Synthesis]
Diphenylacetonitrile
(53) was alkylated with dibromoethane in the presence
of NaNH2 in toluene to give bromide compound 54. The bromide 54 was condensed with 2-methylimidazole
in the presence of Et3N in hot DMF to afford 2-methylimidazole
derivative 55. Hydrolysis of the cyano group of 55
with 70% sulfuric acid provided imidafenacin (VII).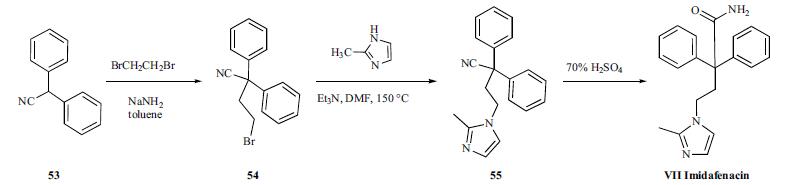
| [storage]
Store at -20°C |
|
|