| Identification | Back Directory | [Name]
Sitaxentan sodium | [CAS]
210421-74-2 | [Synonyms]
Thelin
CS-338
CS-777
TBC-11250
TBC-11249
TBC-11251 sodium
sitaxentan sodiuM
SITAXSENTAN SODIUM
TBC11251 sodiuM salt
Sitaxentan sodium salt
Sitaxsentan sodium salt
Sitaxentan sodium USP/EP/BP
Sitaxsentan sodiuM(TBC-11249)
Sitaxsentan sodiuM (TBC-11251)
Sitaxentan sodium (Sitaxsentan
N-(4-Chloro-3-Methylisoxazol-5-yl)-2-[2-(6-Methyl-1,3-Benzodioxol-5-yl)acetyl]thiophene-3-sulfonaMide
N-(4-Chloro-3-Methyl-5-isoxazolyl)-2-[2-(6-Methyl-1,3-benzodioxol-5-yl)acetyl]-3-thiophenesulfonaMide
4-Chloro-3-methyl-5-[2-[2-(6-methyl-1,3-benzodioxol-5-yl)acetyl]-3-thienylsulfonamido]isoxazole sodium salt
N-(4-Chloro-3-methyl-5-isoxazolyl)-2-[2-(6-methyl-1,3-benzodioxol-5-yl)acetyl]-3-thiophenesulfonamide sodium salt
Sodium (4-chloro-3-methylisoxazol-5-yl)((2-(2-(6-methylbenzo[d][1,3]dioxol-5-yl)acetyl)thiophen-3-yl)sulfonyl)amide
Sodium (4-chloro-3-methyl-1,2-oxazol-5-yl)-[2-[2-(6-methyl-1,3-benzodioxol-5-yl)acetyl]thiophen-3-yl]sulfonylazanide
3-Thiophenesulfonamide, N-(4-chloro-3-methyl-5-isoxazolyl)-2-[2-(6-methyl-1,3-benzodioxol-5-yl)acetyl]-, sodium salt (1:1) | [Molecular Formula]
C18H14ClN2NaO6S2 | [MDL Number]
MFCD11040990 | [MOL File]
210421-74-2.mol | [Molecular Weight]
476.89 |
| Hazard Information | Back Directory | [Description]
In November Encysive Pharmaceuticals launched Thelin®
(sitaxsentan sodium) in the U.K. for the treatment of pulmonary
arterial hypertension (PAH), following European Commission
approval in August 2006. Sitaxsentan is the first
selective endothelin A (ETA) receptor antagonist, and the
first once-daily oral treatment available for patients with
PAH. It is 6,500-fold selective in the targeting of ETA versus ETB receptors. Sitaxsentan is indicated for improving
exercise capacity in PAH patients classified as World Health
Organization (WHO) functional class III. Efficacy has been
shown in primary pulmonary hypertension and pulmonary
hypertension associated with connective tissue disease. In
the U.S., Encysive has submitted a complete response to an
approvable letter received from the FDA in July. | [Description]
Sitaxentan is a potent nonpeptide endothelin A (ETA) receptor antagonist (IC50 = 1.4 nM).1 It is selective for ETA over ETB receptors (IC50 = 9,800 nM). Sitaxentan inhibits phosphoinositol hydrolysis induced by endothelin-1 (Item No. 24127) in COS-7 cells (pA2 = 8). In vivo, sitaxentan reduces blood pressure in a rat model of acute hypoxia-induced pulmonary hypertension (ED50 = 0.5 mg/kg). It reduces femoral artery neointimal lesion size in a mouse model of intraluminal injury.2 Sitaxentan (15 mg/kg) decreases bronchoalveolar lavage fluid (BALF) pleocytosis, as well as pulmonary collagen deposition and fibrosis, and improves lung mechanics in a mouse model of bleomycin-induced lung injury.3 Formulations containing sitaxentan have been used in the treatment of hypertension. | [Uses]
Selective endothelin A (ETA) receptor antagonist. Antihypertensive. Used in treatment of chronic heart failure. | [General Description]
Sitaxentan sodium, belongs to thesulfonamide class of endothelin receptor antagonists.Although it has 6,000-fold selectivity for the ETA receptor,clinical trials have not demonstrated a greater efficacyover bosentan. However, it has much lower liver toxicitythan bosentan. The manufacturer is attempting to meetefficacy outcomes set by the FDA, which must be met beforeapproval of sitaxsentan as a therapeutic agent will begranted.
| [Biochem/physiol Actions]
Sitaxentan (Sitaxsentan) is a potent and selective endothelin ET(A) receptor antagonist once used in the treatment of pulmonary arterial hypertension (PAH), but removed from the market because of hepatotoxicity. It is over 6000-fold selective for the the ETA receptor subtype with an IC50 of 1 nM for ETA versus an IC50 of 9800 nM for ETB. | [Synthesis]
The synthesis
of sitaxentan is depicted in Scheme 12. 5-amino-3-
methylisoxazole 85 was treated with NCS in DCM at 0??C to
give chloroisoxazole 86 in 87% yield. The amine was then
coupled with the commercially available 2-(methoxycarbonyl)-
3-thiophenesulfonyl chloride (87) using sodium hydride
in THF at 0??C. The resulting ester was directly hydrolyzed in
1N NaOH to furnish acid 88 in 45% yield. The acid 88
was then coupled with N,O-dimethylhydroxylamine to give
Weinreb amide 89. The amide 89 was then treated with benzylic
Grignard reagent followed by acidic workup to give the
sitaxentan XII in 50% yield in two steps. The Grignard reagent
90 was prepared through the following sequence. The
5-methylbenzodioxole 91 was treated with aqueous formaldehyde
and concentrated HCl in ethyl ether to give the desired
benzyl chloride 93 and condensation product 92. The
mixture of 92 and 93 was used to form the Grignard reagent
without separation.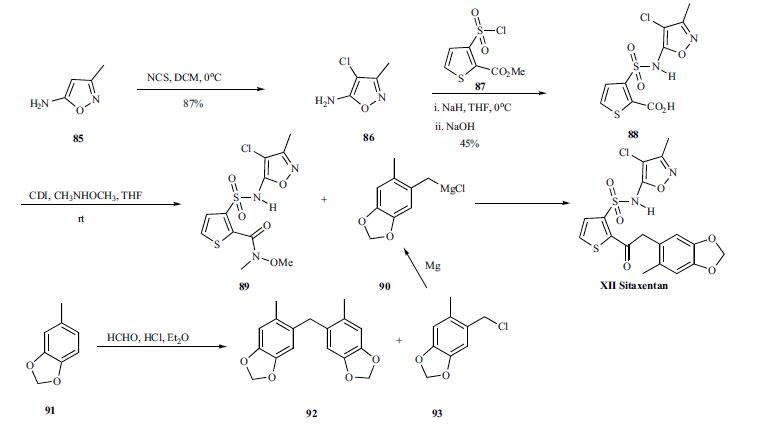
| [storage]
Store at -20°C |
|
|