| Identification | Back Directory | [Name]
Chlortalidone | [CAS]
77-36-1 | [Synonyms]
renon
isoren
oradil
g33182
igroton
oxodolin
zambesil
NSC 6920
hydroton
hygroton
thalitone
saluretin
natriuran
NSC 69200
Clortalidone
chlorthalidon
phthalamodine
phthalamudine
CHLORTALIDONE
CHLORTHALIDONE
chlorothalidone
chlorphthalidone
chlorphthalidolone
CHLORTHALIDONE,USP
Racemic chlorthalidone
Chlorthalidone (200 mg)
3-hydroxy-3-(4-chloro-3-sulfamylphenyl)phthalimidine
3-(4’-chloro-3’-sulfamoylphenyl)-3-hydroxyphthalimidine
1-oxo-3-(3-sulfamyl-4-chlorophenyl)-3-hydroxyisoindoline
3-(4-chloro-3-sulphamoylphenyl)-3-hydroxyisoindolin-1-one
1-(4-Chloro-3-sulphamoylphenyl)-1-hydroxyisoindolin-3-one
1-Oxo-3-(3-sulfamyl-4-chlorophenyl)-3-hydroxy-isoindolinum
1-keto-3-(3’-sulfamyl-4’-chlorophenyl)-3-hydroxyisoindoline
2-Chloro-5-(3-hydroxy-1-oxoisoindol-3-yl)benzenesulfonamide
1-Oxo-3-(4'-chloro-3'-sulphamoylphenyl)-3-hydroxyisoindoline
2-Chloro-5-(1-hydroxy-3-oxoisoindolin-1-yl)benzenesulfonaMide
2-chloro-5-(1-hydroxy-3-oxo-1-isoindolinyl)-benzenesulfonamid
2-Chloro-5-(1-hydroxy-3-oxo-1-isoindolinyl)benzenesulfonamide
2-chloro-5-(1-hydroxy-3-keto-isoindolin-1-yl)benzenesulfonamide
2-chloro-5-(1-hydroxy-3-oxo-2H-isoindol-1-yl)benzenesulfonamide
3-[4-Chloro-3-(sulfamoyl)phenyl]-3-hydroxy-3H-isoindol-1(2H)-one
Benzenesulfonamide, 2-chloro-5-(1-hydroxy-3-oxo-1-isoindolinyl)- (6CI, 8CI)
2-chloro-5-(2,3-dihydro-1-hydroxy-3-oxo-1h-isoindol-1-yl)-benzenesulfonamid
2-chloro-5-(2,3-dihydro-1-hydroxy-3-oxo-1h-isoindol-1-yl)benzenesulfonamide
benzenesulfonamide,2-chloro-5-(2,3-dihydro-1-hydroxy-3-oxo-1h-isoindol-1-yl) | [EINECS(EC#)]
201-022-5 | [Molecular Formula]
C14H11ClN2O4S | [MDL Number]
MFCD00036257 | [MOL File]
77-36-1.mol | [Molecular Weight]
338.77 |
| Hazard Information | Back Directory | [Chemical Properties]
White Solid | [Uses]
Antihypertensive Agents,Diuretics,Sodium Chloride Symporter Inhibitors | [Uses]
Chlorthalidone is used as a diuretic; antihypertensive. | [Originator]
Hygroton, Geigy, US ,1960 | [Definition]
ChEBI: Chlorthalidone is a sulfonamide, a member of isoindoles and a member of monochlorobenzenes. | [Manufacturing Process]
15 parts of aqueous 46% sodium nitrite solution are gradually added to a
mixture of 27.5 parts of 4-chloro-3-amino-benzophenone-2'-carboxylic acid,
200 parts of glacial acetic acid and 20 parts of 37% hydrochloric acid at 0° to
10°C. The solution of the diazonium salt is poured into an ice-cooled mixture
of 200 parts of 30% sulfur dioxide solution in glacial acetic acid and 3 parts of
crystallized cupric chloride in 15 parts of water. Nitrogen is developed and,
after a short time, the 4-chloro-2'-carboxy-benzophenone-3-sulfochloride
crystallizes out. After 1 hour it is filtered off and washed with water. MP 178°
to 182°C.
35.9 parts of 4-chloro-2'-carboxy-benzophenone-3-sulfochloride and 50 parts
of thionyl chloride are heated first for 3 hours at 30° to 35°C and then for 1
hour at 45°C. The excess thionyl chloride is distilled off in the vacuum, the
dichloride, 3-chloro-3-(3'-chlorosulfonyl-4'-chlorophenyl)phthalide, which
remains as a crystallized mass is dissolved in 150 parts of chloroform and a
mixture of 200 parts of 25% aqueous ammonia solution and 200 parts of
ethanol is added dropwise at about 10°C while stirring and cooling. After
stirring for 1 hour at 40°C, the solvent is distilled off in the vacuum and
diluted hydro chloric acid is added to the residue whereupon the 1-oxo-3-(3'-
sulfamyl-4'-chloro-phenyl)3-hydroxy-isoindoline which is tautomeric to the 4-
chloro-2'-carbamyl-benzophenone-3-sulfonamide, separates out. On
recrystallizing from diluted ethanol, the isoindoline derivative melts at 215°C
on decomposition.
Instead of reacting the dichloride in aqueous solution with ammonia, it can
also be reacted at -50° to -40°C with a great excess of liquid ammonia. After
removal of the ammonia, the crude product obtained is recrystallized as
described above. | [Brand name]
Hygroton (Sanofi Aventis); Thalitone
(Monarch). | [Therapeutic Function]
Diuretic, Antihypertensive | [Biochem/physiol Actions]
Chlorthalidone is a thiazide-like diuretic, an inhibitor of the Na+-Cl- cotransporter. Chlorthalidone inhibits sodium ion transport across the renal tubular epithelium increasing the delivery of sodium to the distal renal tubule and indirectly increasing potassium excretion via the sodium-potassium exchange mechanism. Chlorthalidone also promotes Ca++ reabsorption by an unknown mechanism. Several recent comparison studies inidcate that chlorthalidone may be a better drug in preventing cardiovascular events than hydrochlorothiazide. | [Clinical Use]
Chlorthalidone has a long duration of action (48–72 hours). Although quinethazone and metolazone are
administered daily, chlorthalidone may be administered in doses of 25 to 100 mg three times a week. When chlorthalidone is formulated with the excipient povidone, the
product, Thalitone, has greater bioavailability (>90%) and reaches peak plasma concentrations in a shorter time compared with its other products. Similar to the
quinazolinones, it also is extensively bound to carbonic anhydrase in the erythrocytes. | [Synthesis]
Chlorothalidone, 2-chloro-5-(1-hydroxy-3-oxo-1-isoindolinyl)benzolsulfamide
(21.3.26), is synthesized by two proposed methods from 2�-carboxy-4-chlorobenzophenone
(21.3.21), which is easily synthesized by acylating chlorobenzol with phthalic
anhydride in the presence of aluminum chloride. The resulting benzophenone (21.3.21) undergoes nitration by nitric acid, which gives 2�-carboxy-3-nitro-4-chlorobenzophenone
(21.3.22). The nitro group in the resulting compound is reduced by tin dichloride to 2�-
carboxy-3-amino-4-chlorobenzophenone (21.3.23). Next, subsequent diazotation and reaction
with sulfur dioxide in the presence of copper dichloride gives the corresponding sulfonylchloride
(21.3.24). Upon reaction with thionyl chloride, this compound undergoes
cyclization into phtahlide (21.3.25), which when reacted with aqueous ammonia rearranges
into a derivative of isoindoline with simultaneous substitution of the chloride atom in the sulfogroup
with an amino group, which results in chlorothalidone (21.3.26).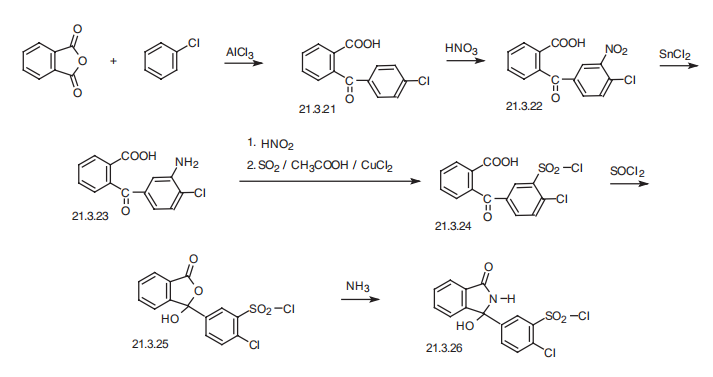
| [Drug interactions]
Potentially hazardous interactions with other drugs
Analgesics: increased risk of nephrotoxicity with
NSAIDs; antagonism of diuretic effect.Anti-arrhythmics: hypokalaemia leads to increased
cardiac toxicity; effects of lidocaine and mexiletine
antagonised.
Antibacterials: avoid administration with
lymecycline.
Antidepressants: increased risk of hypokalaemia with
reboxetine; enhanced hypotensive effect with MAOIs;
increased risk of postural hypotension with tricyclics.
Antiepileptics: increased risk of hyponatraemia with
carbamazepine.
Antifungals: increased risk of hypokalaemia with
amphotericin.
Antihypertensives: enhanced hypotensive effect;
increased risk of first dose hypotension with post�synaptic alpha-blockers like prazosin; hypokalaemia
increases risk of ventricular arrhythmias with sotalol.
Antipsychotics: hypokalaemia increases risk
of ventricular arrhythmias with amisulpride;
enhanced hypotensive effect with phenothiazines;
hypokalaemia increases risk of ventricular
arrhythmias with pimozide - avoid.
Atomoxetine: hypokalaemia increases risk of
ventricular arrhythmias.
Cardiac glycosides: increased toxicity if hypokalaemia
occurs.
Ciclosporin: increased risk of nephrotoxicity and
hypomagnesaemia.
Cytotoxics: increased risk of ventricular arrhythmias
due to hypokalaemia with arsenic trioxide; increased
risk of nephrotoxicity and ototoxicity with platinum
compounds.
Lithium excretion reduced, increased toxicity. | [Metabolism]
Chlortalidone is highly bound to red blood cells; the
receptor to which it is bound has been identified as carbonic
anhydrase. It is much less strongly bound to plasma proteins.
Chlortalidone is mainly excreted unchanged in the urine. | [storage]
Store at -20°C |
|
|