1,3,4-oxadiazole Chemische Eigenschaften,Einsatz,Produktion Methoden
Beschreibung
1,3,4-Oxadiazole is a five-membered, conjugated, planar, stable heteroaromatic, comprised of two adjacent nitrogen atoms at the 3,4-positions with one oxygen atom and two vicinal carbon atoms. Each nitrogen atom in the ring is one carbon apart from the oxygen heteroatom. The 1,3,4-oxadiazole ring is electron deficient due to the presence of two pyridine-like nitrogen atoms in the ring and as a result its chemical properties are unlike those of furan as evidenced from the downfield chemical shift of C2(5)H at δ 8.73 ppm (CDCl3) of the parent 1,3,4-oxadiazole. The presence of two electronegative nitrogen atoms deactivates the ring, making electrophilic substitution difficult in the ring at the C2- and C5-positions. From a medicinal chemistry point of view, it is a very important molecule because it is a surrogate of carboxylic acids, esters, and carboxamides and displays a wide spectrum of pharmacological activities.
Physikalische Eigenschaften
The parent 1,3,4-oxadiazole is a liquid with a bp of 150°C, is soluble in water, and varies with substituents. The presence of hydrophobic groups decreases the solubility in water. Replacement of the alkyl substituent by the aryl group increases the bp and mp of the compounds.
Verwenden
1,3,4-Oxadiazole has therapeutic potential with antibacterial, anti-?mycobacterial, antitumor, anti-?viral and antioxidant activity.
Application
Diverse pharmacological activities such as bactericidal, fungicidal, antiinflammatory, sedative, analgesic, antidepressant, antiproteolytic, anesthetic, and anticonvulsant are associated with diaryl- and amino-1,3,4-oxadiazoles. Some of the oxadiazole derivatives such as oxadiazolinones have shown insecticidal and herbicidal activities. One of the oxadiazole derivatives, (2-[4-biphenylyl]-5-[4-tert-butylphenyl])-1,3,4-oxadiazole, is in diagnostic use as a scintillator. 2,5-Disubstituted-1,3,4-oxadiazoles generally produce fluorescence and are used as laser dyes, optical brighteners, scintillators, or electrophotographic photoconductors. 2,5-Dipicryl-1,3,4-oxadiazole has been reported as an explosive. Corrosion produced in mild steel by acid solution has been inhibited by 2-aryl-5-oxadiazoline-5-thiones. A series of liquid crystals based on 1,3,4-oxadiazoles has been prepared and used to study the flexoelectric effect in guest/host mixtures.
synthetische
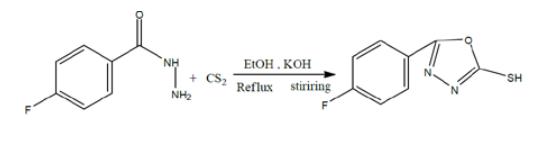
A solution of potassium hydroxide (4 pellets) was made in absolute ethanol (40ml) & poured into a round bottom flask. 10g of prepared hydrazide and 10ml of carbon disulphide was added in the round bottom flask. Condenser was adjusted and allowed to reflux for 6-8 hours. Reaction progress was checked at regular intervals by using TLC procedure with the use of varying ratio of n-hexane & ethyl acetate. As the reaction got completed, 20ml of chilled distilled water and a very small amount of dil. Sulfuric acid (H2SO4) to maintain the pH 2-3 in order to remove un-reacted base. Solid precipitates were obtained on vigorous shaking and filtered. Product was dried, collected &calculated.
Chemische Reaktivität
Electrophilic substitution to 1,3,4-oxadiazole is unusual because electron density at the C2-and C5-positions is low. Electrophilic substitution is facile in aryl group present as substituent in lieu of 1,3,4-oxadiazole ring. Nucleophilic substitution reactions are uncommon in 1,3,4-oxadiazoles but ring cleavage is very common.
1,3,4-oxadiazole Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

