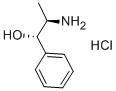
DL-Norephedrine hydrochloride
- CAS No.
- 154-41-6
- Chemical Name:
- DL-Norephedrine hydrochloride
- Synonyms
- ip58064;obestat;monydrin;mucorama;EOS-60988;NSC 23798;NOREPHEDRINE HCL;dl-NOREPHEDRINE HCl;LincomycinHydrocloride;PHENYLPROPANOLAMINE HCL
- CBNumber:
- CB0304191
- Molecular Formula:
- C9H14ClNO
- Molecular Weight:
- 187.67
- MDL Number:
- MFCD00013019
- MOL File:
- 154-41-6.mol
| Melting point | 194-196 °C(lit.) |
|---|---|
| Density | 1.0969 (rough estimate) |
| refractive index | 1.5330 (estimate) |
| storage temp. | -20°C |
| solubility | Dissolve 1.25 g in water R and dilute to 25 mL with the same solvent. |
| form | A crystalline solid |
| pka | 9.44(at 25℃) |
| Merck | 13,7391 |
| CAS DataBase Reference | 154-41-6(CAS DataBase Reference) |
| FDA UNII | 8D5I63UE1Q |
| EPA Substance Registry System | Benzenemethanol, .alpha.-[(1R)-1-aminoethyl]-, hydrochloride (1:1), (.alpha.S)-rel- (154-41-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302 | |||||||||
| Precautionary statements | P264-P270-P301+P312-P330-P501 | |||||||||
| Hazard Codes | Xn,T,F | |||||||||
| Risk Statements | 22-39/23/24/25-23/24/25-11 | |||||||||
| Safety Statements | 22-36/37/39-45-36/37-16-7 | |||||||||
| RIDADR | UN 2811 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | DN4200000 | |||||||||
| HS Code | 29394900 | |||||||||
| Toxicity | LD50 orally in rats: 1490 mg/kg (Goldenthal) | |||||||||
| NFPA 704 |
|
DL-Norephedrine hydrochloride Chemical Properties,Uses,Production
Chemical Properties
white crystalline powder. soluble in water and alcohol, insoluble in ether, chloroform and benzene.
Originator
Propadrine, MSD ,US ,1941
Uses
DL-Norephedrine is a psychoactive amphetamine that acts as a potent norepinephrine releasing agent (EC50 = 42-137 nM). It is less effective as a dopamine releasing agent (EC50 = 0.3-1.4 μM) and does not affect serotonin release. This product is intended for forensic and research applications.[Cayman Chemical]
Manufacturing Process
In one route as described in US Patent 2,151,517, 10.7 kg of technical benzaldehyde is vigorously agitated with a solution of 11.0 kg of sodium bisulfite in 50.0 liters of water until the formation of the addition-product is complete. Simultaneously, 8.25 kg of nitroethane is dissolved in a solution of 4.5 kg of caustic soda in 20.0 liters of water and the resultant warm solution is added with vigorous stirring to the magma of benzaldehyde sodium bisulfite. The mixture is agitated for 30 minutes and then allowed to stand overnight.
The aqueous portion of the mixture is now siphoned off from the supernatant layer of oily phenylnitropropanol and replaced with a fresh solution of 11.0 kg of sodium bisulfite in 50.0 liters of water. The mixture of phenylnitropropanol and bisulfite solution is now vigorously agitated for 15 minutes in order to remove and recover small amounts of unreacted benzaldehyde, and is then again allowed to stratify. This time, the phenylnitropropanol is siphoned off and filtered to remove a small amount of resinous material. The aqueous solution of sodium bisulfite remaining behind is reacted with benzaldehyde, as described above, thus making the process continuous.
The 1-phenyl-2-nitropropanol thus obtained is a colorless oil, specific gravity 1.14 at 20°C, odorless when pure, volatile with steam and boiling at 150° to 165°C under a pressure of 5 mm of mercury. It is soluble in alcohol, ether, acetone, chloroform, carbon tetrachloride, benzene and glacial acetic acid. The yield of 1-phenyl-2-nitropropanolobtained by this procedure is 17.1 to 17.7 kg.
It is hydrogenated and converted to the hydrochloride in subsequent steps. The hydrogen chloride has a melting point of 192°-194°C.
In an alternative route described in US Patent 3,028,429 propiophenone may be reacted with an alkyl nitrite to give isonitrosopropiophenone which is then hydrogenated and finally converted to the hydrochloride.
Therapeutic Function
Nasal decongestant, Anorexic
General Description
Phenylpropanolamine hydrochloride is an odorless white to creamy-white crystalline powder. Bitter taste. pH (3% solution):4.5-6. pH (10% solution) 4.2-5.5. (NTP, 1992)
Air & Water Reactions
Water soluble.
Reactivity Profile
DL-Norephedrine hydrochloride is incompatible with strong oxidizing agents.
Hazard
Poison by ingestion.
Fire Hazard
Flash point data for DL-Norephedrine hydrochloride are not available; however, DL-Norephedrine hydrochloride is probably combustible.
Safety Profile
Poison by ingestion, subcutaneous, intravenous, intraperitoneal, and intramuscular routes. An experimental teratogen. When heated to decomposition it emits very toxic fumes of HCl and NOx. Used as a raw material in cold and diet tablets.
DL-Norephedrine hydrochloride Preparation Products And Raw materials
Raw materials
1of2