Hydrogen
- CAS No.
- 1333-74-0
- Chemical Name:
- Hydrogen
- Synonyms
- H2;Dihydrogen;Wasserstoff;Liquid hydrogen;Molecular hydrogen;HYDROGE;PROTIUM;HYDROGEN;diprotium;o-Hydrogen
- CBNumber:
- CB7686195
- Molecular Formula:
- H2
Lewis structure
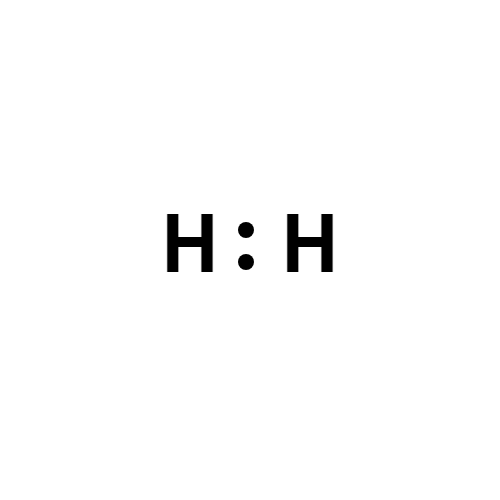
- Molecular Weight:
- 2.02
- MDL Number:
- MFCD00070838
- MOL File:
- 1333-74-0.mol
- MSDS File:
- SDS
| Melting point | −259.2 °C(lit.) |
|---|---|
| Boiling point | −252.8 °C(lit.) |
| Density | 0.0899 |
| vapor density | 0.07 (21 °C, vs air) |
| vapor pressure | Critical temperature is - 239.9 °C; noncondensible above this temperature |
| Flash point | <-150°C |
| solubility | slightly soluble in H2O |
| pka | 35(at 25℃) |
| form | colorless gas |
| color | colorless gas; flammable |
| Odor | Odorless gas |
| explosive limit | 74.2% |
| Water Solubility | 0.00017 g/100 mL |
| Merck | 13,4813 |
| Dielectric constant | 1.0(100℃) |
| Stability | Stable. Highly flammable. Readily forms explosive mixtures with air. Upper (U.K.) composition limit for use of a nitrogen/hydrogen mixture in the open lab is 5.7% hydrogen. |
| CAS DataBase Reference | 1333-74-0(CAS DataBase Reference) |
| Indirect Additives used in Food Contact Substances | HYDROGEN |
| FDA 21 CFR | 175.105; 177.2800 |
| EWG's Food Scores | 1 |
| FDA UNII | 7YNJ3PO35Z |
| NIST Chemistry Reference | Hydrogen(1333-74-0) |
| EPA Substance Registry System | Hydrogen (1333-74-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS04 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H220-H280 | |||||||||
| Precautionary statements | P210-P377-P381-P410+P403 | |||||||||
| Hazard Codes | F+ | |||||||||
| Risk Statements | 12 | |||||||||
| Safety Statements | 9-16-33 | |||||||||
| RIDADR | UN 1950 2.1 | |||||||||
| WGK Germany | - | |||||||||
| RTECS | MW8900000 | |||||||||
| F | 4.5-31 | |||||||||
| Autoignition Temperature | 500 to 590 °C | |||||||||
| DOT Classification | 2.1 (Flammable gas) | |||||||||
| HazardClass | 2.1 | |||||||||
| Toxicity | TLV-TWA (ACGIH) None established; simple asphyxiant | |||||||||
| NFPA 704 |
|
Hydrogen price More Price(4)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 295396 | Hydrogen ≥99.99% | 1333-74-0 | 56l | $581 | 2024-03-01 | Buy |
| Sigma-Aldrich | 295396 | Hydrogen ≥99.99% | 1333-74-0 | 57l | $614 | 2024-03-01 | Buy |
| American Custom Chemicals Corporation | CHM0030059 | HYDROGEN 95.00% | 1333-74-0 | 56L | $6551.74 | 2021-12-16 | Buy |
| Oakwood | 101507 | Hydrogen | 1333-74-0 | 56L | $190 | 2021-12-16 | Buy |
Hydrogen Chemical Properties,Uses,Production
Description
Hydrogen is colorless, odorless, tasteless, flammable, and nontoxic. It exists as a gas at ambient temperatures and atmospheric pressures. It is the lightest gas known, with a density approximately 0.07% that of air. Hydrogen is present in the atmosphere occurring in concentrations of only about 0.5 ppm by volume at lower altitudes.
Chemical Properties
Hydrogen,H2, is a tasteless,colorless, odorless gas that may be liquified by cooling under pressure. Hydrogen is used in welding, in the production of ammonia, methanol, and other chemicals, for the hydrogenation of oil and coal,and for the reduction of metallic oxide ores.It is obtained by the dissociation of water and as a by-product in the electrolysis of brine solutions. Molecular hydrogen at ambient temperature is relatively innocuous to most metals.However, atomic hydrogen is detrimental to most metals.
Physical properties
Hydrogen’s atom is the simplest of all the elements, and the major isotope (H-1) consists ofonly one proton in its nucleus and one electron in its K shell. The density of atomic hydrogenis 0.08988 g/l, and air’s density is 1.0 g/l (grams per liter). Its melting point is –255.34°C,and its boiling point is –252.87°C (absolute zero = –273.13°C or –459.4°F). Hydrogen hastwo oxidation states, +1 and –1.
Isotopes
The major isotope of hydrogen has just one proton and no neutrons in itsnucleus (1H-1).Deuterium (2D or H-2) has a nucleus consisting of one proton plus one neutron. Tritium (3T or H-3), another variety of heavy water (TOT),has nuclei consisting of one proton and two neutrons.
Origin of Name
Hydrogen was named after the Greek term hydro genes, which means “water former.”
Characteristics
H2 is a diatomic gas molecule composed of two tightly joined atoms that strongly sharetheir outer electrons. It is an odorless, tasteless, and colorless gas lighter than air. Hydrogenis included in group 1 with the alkali metals because it has an oxidation state of +1 as dothe other alkali metals. Experiments during the 1990s at the Lawrence Livermore NationalLaboratory (LLNL), in Livermore, California, lowered the temperature of H2 to almostabsolute zero. By exploding gunpowder in a long tube that contained gaseous hydrogen, thegas that was under pressure of over one million times the normal atmospheric pressure wascompressed into a liquid. This extreme pressure on the very cold gas converted it to liquidhydrogen (almost to the point of solid metallic hydrogen), in which state it did act as a metaland conduct electricity.Hydrogen gas is slightly soluble in water, alcohol, and ether. Although it is noncorrosive,it can permeate solids better than air. Hydrogen has excellent adsorption capabilities in theway it attaches and holds to the surface of some substances. (Adsorption is not the same asabsorption with a “b,” in which one substance intersperses another.
History
Hydrogen was prepared many years before it was recognized as a distinct substance by Cavendish in 1766. It was named by Lavoisier. Hydrogen is the most abundant of all elements in the universe, and it is thought that the heavier elements were, and still are, being built from hydrogen and helium. It has been estimated that hydrogen makes up more than 90% of all the atoms or three quarters of the mass of the universe. It is found in the sun and most stars, and plays an important part in the proton– proton reaction and carbon–nitrogen cycle, which accounts for the energy of the sun and stars. It is thought that hydrogen is a major component of the planet Jupiter and that at some depth in the planet’s interior the pressure is so great that solid molecular hydrogen is converted into solid metallic hydrogen. In 1973, it was reported that a group of Russian experimenters may have produced metallic hydrogen at a pressure of 2.8 Mbar. At the transition the density changed from 1.08 to 1.3 g/cm3. Earlier, in 1972, a Livermore (California) group also reported on a similar experiment in which they observed a pressure-volume point centered at 2 Mbar. It has been predicted that metallic hydrogen may be metastable; others have predicted it would be a superconductor at room temperature. On Earth, hydrogen occurs chiefly in combination with oxygen in water, but it is also present in organic matter such as living plants, petroleum, coal, etc. It is present as the free element in the atmosphere, but only to the extent of less than 1 ppm by volume. It is the lightest of all gases, and combines with other elements, sometimes explosively, to form compounds. Great quantities of hydrogen are required commercially for the fixation of nitrogen from the air in the Haber ammonia process and for the hydrogenation of fats and oils. It is also used in large quantities in methanol production, in hydrodealkylation, hydrocracking, and hydrodesulfurization. It is also used as a rocket fuel, for welding, for production of hydrochloric acid, for the reduction of metallic ores, and for filling balloons. The lifting power of 1 ft3 of hydrogen gas is about 0.076 lb at 0°C, 760 mm pressure. Production of hydrogen in the U.S. alone now amounts to about 3 billion cubic feet per year. It is prepared by the action of steam on heated carbon, by decomposition of certain hydrocarbons with heat, by the electrolysis of water, or by the displacement from acids by certain metals. It is also produced by the action of sodium or potassium hydroxide on aluminum. Liquid hydrogen is important in cryogenics and in the study of superconductivity, as its melting point is only a 20°C above absolute zero. Hydrogen consists of three isotopes, most of which is 1H. The ordinary isotope of hydrogen, H, is known as protium. In 1932, Urey announced the discovery of a stable isotope, deuterium (2H or D) with an atomic weight of 2. Deuterium is present in natural hydrogen to the extent of 0.015%. Two years later an unstable isotope, tritium (3H), with an atomic weight of 3 was discovered. Tritium has a half-lifeof about 12.32 years. Tritium atoms are also present in natural hydrogen but in a much smaller proportion. Tritium is readily produced in nuclear reactors and is used in the production of the hydrogen bomb. It is also used as a radioactive agent in making luminous paints, and as a tracer. On August 27, 2001 Russian, French, and Japanese physicists working at the Joint Institute for Nuclear Research near Moscow reported they had made “super-heavy hydrogen,” which had a nucleus with one proton and four neutrons. Using an accelerator, they used a beam of helium-6 nuclei to strike a hydrogen target, which resulted in the occasional production of a hydrogen-5 nucleus plus a helium-2 nucleus. These unstable particles quickly disintegrated. This resulted in two protons from the He-2, a triton, and two neutrons from the H-5 breakup. Deuterium gas is readily available, without permit, at about $1/l.
Uses
In oxy-hydrogen blowpipe (welding) and limelight; autogenous welding of steel and other metals; manufacture of ammonia, synthetic methanol, HCl, NH3; hydrogenation of oils, fats, naphthalene, phenol; in balloons and airships; in metallurgy to reduce oxides to metals; in petroleum refining; in thermonuclear reactions (ionizes to form protons, deuterons (D) or tritons (T)). liquid hydrogen used in bubble chambers to study subatomic particles; as a coolant.
Uses
Large quantities of hydrogen are produced on
site or pipelined for use by refineries, petrochemical
and bulk chemical facilities for hydrotreating,
catalytic reforming, and hydrocracking.
Smaller quantities of hydrogen are
produced on site or pipelined for use in the
chemical, metallurgical, fats and oils, glass, and
electronic industries.
Some of these smaller users have hydrogen
delivered to their manufacturing location as
gaseous hydrogen in cylinders or tube trailers,
or by cascade into on-site storage cylinders.
Certain smaller users have liquid hydrogen delivered
into an on-site liquid hydrogen storage
system.
Uses
Hydrogen is an excellent reducing agent.Production of ammonia (NH3).Ethanol (ethyl alcohol made from grains).Hydrogenation of vegetable oils.
Definition
hydrogen: Symbol H. A colourlessodourless gaseous chemical element;a.n. 1; r.a.m. 1.008; d. 0.0899 g dm–3;m.p. –259.14°C; b.p. –252.87°C. It isthe lightest element and the mostabundant in the universe. It is presentin water and in all organic compounds.There are three isotopes:naturally occurring hydrogen consistsof the two stable isotopes hydrogen–1 (99.985%) and deuterium. Theradioactive tritium is made artificially.The gas is diatomic and hastwo forms: orthohydrogen, in whichthe nuclear spins are parallel, andparahydrogen, in which they are antiparallel.At normal temperaturesthe gas is 25% parahydrogen. In theliquid it is 99.8% parahydrogen. Themain source of hydrogen is steamreforming of natural gas. It can alsobe made by the Bosch process (seehaber process) and by electrolysis ofwater. The main use is in the Haberprocess for making ammonia. Hydrogenis also used in various other industrial processes, such as thereduction of oxide ores, the refiningof petroleum, the production ofhydrocarbons from coal, and the hydrogenationof vegetable oils. Considerableinterest has also been shownin its potential use in a ‘hydrogenfuel economy’ in which primary energysources not based on fossil fuels(e.g. nuclear, solar, or geothermal energy)are used to produce electricity,which is employed in electrolysingwater. The hydrogen formed isstored as liquid hydrogen or as metalhydrides. Chemically, hydrogen reactswith most elements. It was discoveredby Henry Cavendish in1766.
Production Methods
Hydrogen gas may be produced by several methods. It is commerciallyobtained by electrolysis of water. It also is made industrially by the reactionof steam with methane or coke:
CH4 + H2O → CO + 3H2
C + H2O → CO + H2
CO + H2O → CO2 + H2
The reactions are carried out at about 900 to 1,000°C and catalyzed by nick-el, nickel-alumina, or rhodium-alimina catalysts. In the laboratory, hydrogenmay be prepared by the reaction of zinc or iron with dilute hydrochloric or sulfuric acid:
Zn + 2HCl → ZnCl2 + H2
It also may be prepared by passing water vapor over heated iron:
H2O + Fe → FeO + H2
Also, it can be generated by reaction of metal hydrides with water:
CaH2 + 2H2O → Ca(OH)2 + 2H2
Another method of preparation involves heating aluminum, zinc, or otheractive metals in dilute sodium hydroxide or potassium hydroxide:
2Al + 6NaOH → 2Na3AlO3 + 3H2
Zn + 2KOH → K2ZnO2 + H2
Definition
ChEBI: An elemental molecule consisting of two hydrogens joined by a single bond.
General Description
Hydrogen is a colorless, odorless gas. Hydrogen is easily ignited. Once ignited Hydrogen burns with a pale blue, almost invisible flame. The vapors are lighter than air. Hydrogen is flammable over a wide range of vapor/air concentrations. Hydrogen is not toxic but is a simple asphyxiate by the displacement of oxygen in the air. Under prolonged exposure to fire or intense heat the containers may rupture violently and rocket. Hydrogen is used to make other chemicals and in oxyHydrogen welding and cutting.
Air & Water Reactions
Highly flammable.
Reactivity Profile
Finely divided platinum and some other metals will cause a mixture of Hydrogen and oxygen to explode at ordinary temperatures. If a jet of Hydrogen in air impinges on platinum black the metal surface gets hot enough to ignite the gases, [Mellor 1:325(1946-1947)]. Explosive reactions occur upon ignition of mixtures of nitrogen trifluoride with good reducing agents such as ammonia, Hydrogen, Hydrogen sulfide or methane. Mixtures of Hydrogen, carbon monoxide, or methane and oxygen difluoride are exploded when a spark is discharged, [Mellor 2, Supp. 1:192(1956)]. An explosion occurred upon heating 1'-pentol and 1''-pentol under Hydrogen pressure. Hydrogen appears that this acetylenic compound under certain conditions suddenly breaks down to form elemental carbon, Hydrogen, and carbon monoxide with the release of sufficient energy to develop pressures in excess of 1000 atmospheres, [AIChE Loss Prevention, p1, (1967)].
Hazard
Highly flammable and explosive, dangerous when exposed to heat or flame, explosive limits in air 4–75% by volume.
Hazard
Hydrogen gas is very explosive when mixed with oxygen gas and touched off by a spark or flame. Many hydrides of hydrogen are dangerous and can become explosive if not stored and handled correctly. Many organic and hydrocarbon compounds are essential for life to exist, but just as many are poisonous, carcinogenic, or toxic to living organisms.
Health Hazard
Hydrogen is practically nontoxic. In high concentrations this gas is a simple asphyxiant, and ultimate loss of consciousness may occur when oxygen
Health Hazard
Vapors may cause dizziness or asphyxiation without warning. Some may be irritating if inhaled at high concentrations. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire may produce irritating and/or toxic gases.
Fire Hazard
EXTREMELY FLAMMABLE. Will be easily ignited by heat, sparks or flames. Will form explosive mixtures with air. Vapors from liquefied gas are initially heavier than air and spread along ground. CAUTION: Hydrogen (UN1049), Deuterium (UN1957), Hydrogen, refrigerated liquid (UN1966) and Methane (UN1971) are lighter than air and will rise. Hydrogen and Deuterium fires are difficult to detect since they burn with an invisible flame. Use an alternate method of detection (thermal camera, broom handle, etc.) Vapors may travel to source of ignition and flash back. Cylinders exposed to fire may vent and release flammable gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket.
Fire Hazard
Hydrogen is a highly flammable gas that burns with an almost invisible flame and low heat radiation. Hydrogen forms explosive mixtures with air from 4 to 75% by volume. These explosive mixtures of hydrogen with air (or oxygen) can be ignited by a number of finely divided metals (such as common hydrogenation catalysts). In the event of fire, shut off the flow of gas and extinguish with carbon dioxide, dry chemical, or halon extinguishers. Warming of liquid hydrogen contained in an
Flammability and Explosibility
Hydrogen is a highly flammable gas that burns with an almost invisible flame and low heat radiation. Hydrogen forms explosive mixtures with air from 4 to 75% by volume. These explosive mixtures of hydrogen with air (or oxygen) can be ignited by a number of finely divided metals (such as common hydrogenation catalysts). In the event of fire, shut off the flow of gas and extinguish with carbon dioxide, dry chemical, or halon extinguishers. Warming of liquid hydrogen contained in an enclosed vessel to above its critical temperature can cause bursting of that container.
Agricultural Uses
Hydrogen, a non-metallic element, is a colorless odorless, tasteless gas occurring in water combined with oxygen, and in all organic compounds (for example, hydrocarbons and carbohydrates). It is produced by electrolysis of water and is used in the Haber-Bosch process for producing ammonia - a major raw material for nitrogenous fertilizers.
Large quantities of hydrogen are utilized in catalytic hydrogenation of unsaturated vegetable oils to make solid fats and petroleum refining. Large quantities of hydrogen are also used as a propulsion fuel for rockets in conjunction with oxygen or fluorine. Being flammable, it is used with helium for filling balloons and airships.
Hydrogen is the lightest of all the elements holding position in Group 1 of the Periodic Table. It is abundant in the universe. There are three hydrogen isotopes namely hydrogen- 1, deuterium and tritium. The first two are naturally occurring stable isotopes and the third being radioactive, is made artificially.
Materials Uses
Hydrogen gas is noncorrosive and may be contained
at ambient temperatures by most common
metals used in installations designed to have
sufficient strength for the working pressures
involved. Equipment and piping built to use
hydrogen should be selected with consideration
of the possibility of embrittlement, particularly
at elevated pressures and temperatures above
450°F (232°C). A Nelson curve should be consulted
to select the proper alloys.
Metals used for liquid hydrogen equipment
must have satisfactory properties at very low
operating temperatures. Ordinary carbon steels
lose their ductility at liquid hydrogen temperatures
and are considered too brittle for this
service. Suitable materials include austenitic
chromium-nickel steels (stainless steels), copper,
copper silicon alloys, aluminum, Monel,
and some brasses and bronzes.
Potential Exposure
Hydrogen is used as a fuel in weeding,as a raw material for ammonia manufacture, and in organichydrogenation reactions.
Physiological effects
Hydrogen is nontoxic, but it can act as a simple asphyxiant by displacing or diluting atmospheric air to the point where the oxygen content cannot support life. Unconsciousness without any warning symptoms can occur from inhaling air that contains a sufficiently large amount of hydrogen.
First aid
Contact With Liquid Hydrogen: Put affected partinto warm water. Seek medical attention. Breathing:Remove the person from exposure. Begin (using universalprecautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility.
storage
hydrogen cylinders should be clamped or otherwise supported in place and used only in areas free of ignition sources and separate from oxidizers. Expansion of hydrogen released rapidly from a compressed cylinder will cause evolution of heat due to its negative Joule-Thompson coefficient.
Shipping
Hydrogen, compressed, requires a shipping labelof“FLAMMABLE GAS"”It falls in Hazard Class 2.1.Hydrogen, refrigerated liquid, requires a shipping label of“FLAMMABLE GAS." It falls in Hazard Class 2.1.
Purification Methods
It is usually purified by passing through a suitable absorption train of tubes. Carbon dioxide is removed with KOH pellets, soda-lime or NaOH pellets. Oxygen is removed with a “De-oxo” unit or by passage over Cu heated to 450-500o and Cu on Kieselguhr at 250o. Passage over a mixture of MnO2 and CuO (Hopcalite) oxidises any CO to CO2 (which is removed as above). Hydrogen can be dried by passage through dried silica-alumina at -195o, through a dry-ice trap followed by a liquid-N2 trap packed with glass wool, through CaCl2 tubes, or through Mg(ClO4)2 or P2O5. Other purification steps include passage through a hot palladium thimble [Masson J Am Chem Soc 74 4731 1952], through an activated-charcoal trap at -195o, and through a non-absorbent cotton-wool filter or small glass spheres coated with a thin layer of silicone grease. Potentially VERY EXPLOSIVE in air.
Incompatibilities
Hydrogen is a reducing agent and reacts explosively with strong oxidizers such as halogens (fluorine, chlorine, bromine, iodine) and interhalogen compounds.
Waste Disposal
Excess hydrogen cylinders should be returned to the vendor. Excess hydrogen gas present over reaction mixtures should be carefully vented to the atmosphere under conditions of good ventilation after all ignition sources have been removed. For more information on disposal procedures, see Chapter 7 of this volume.
GRADES AVAILABLE
CGA G-5.3, Commodity Specification for Hydrogen, presents the component maxima in parts per million (v/v), unless shown otherwise, for the types and grades of hydrogen [1]. These are also known as quality verification levels (QVLs). Gaseous hydrogen is denoted as Type I, and liquefied hydrogen as Type II in the table. A blank indicates no maximum limiting characteristics are specified.
Hydrogen Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of8
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Shanghai Longyu Biotechnology Co., Ltd. | +8615821988213 | info@longyupharma.com | China | 2531 | 58 |
| Hebei shuoxi biotechnology co. LTD | +8613081092107 | CHINA | 968 | 58 | |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49390 | 58 |
| Hebei Binshare New Material Co. Ltd | +8618633865755 | china01@hbbinshare.com | CHINA | 969 | 58 |
| Career Henan Chemica Co | +86-0371-86658258 15093356674; | laboratory@coreychem.com | China | 30255 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| Hebei Zhanyao Biotechnology Co. Ltd | 15369953316 +8615369953316 | admin@zhanyaobio.com | China | 2136 | 58 |
Related articles
- Hydrogen:Chemistry,Preparation,Uses
- Under normal conditions on Earth, elemental hydrogen exists as the diatomic gas, H2. Yet, hydrogen gas is very rare in the Ear....
- Feb 20,2023
- Hydrogen-Hazard and Toxicity
- Hydrogen,H2, is a tasteless,colorless, odorless gas that may be liquified by cooling under pressure. Hydrogen is used in weldi....
- Sep 9,2019
View Lastest Price from Hydrogen manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-05-04 | Hydrogen gas
1333-74-0
|
US $48.00-40.00 / KG | 1KG | 99 | 2tons | Guangzhou Sunton Biotechnology Co., Ltd. | |
 |
2023-04-17 | Hydrogen
1333-74-0
|
US $1.00 / ASSAYS | 1ASSAYS | 99% | 100ton | Shanghai Yunao International Trade Co., Ltd | |
 |
2021-09-07 | Hydrogen gas
1333-74-0
|
US $0.00 / KG | 1KG | Above 99% | 10000kgs | Hebei Zhanyao Biotechnology Co. Ltd |
-

- Hydrogen gas
1333-74-0
- US $48.00-40.00 / KG
- 99
- Guangzhou Sunton Biotechnology Co., Ltd.
-

- Hydrogen
1333-74-0
- US $1.00 / ASSAYS
- 99%
- Shanghai Yunao International Trade Co., Ltd
-

- Hydrogen gas
1333-74-0
- US $0.00 / KG
- Above 99%
- Hebei Zhanyao Biotechnology Co. Ltd
1333-74-0(Hydrogen)Related Search:
1of5





