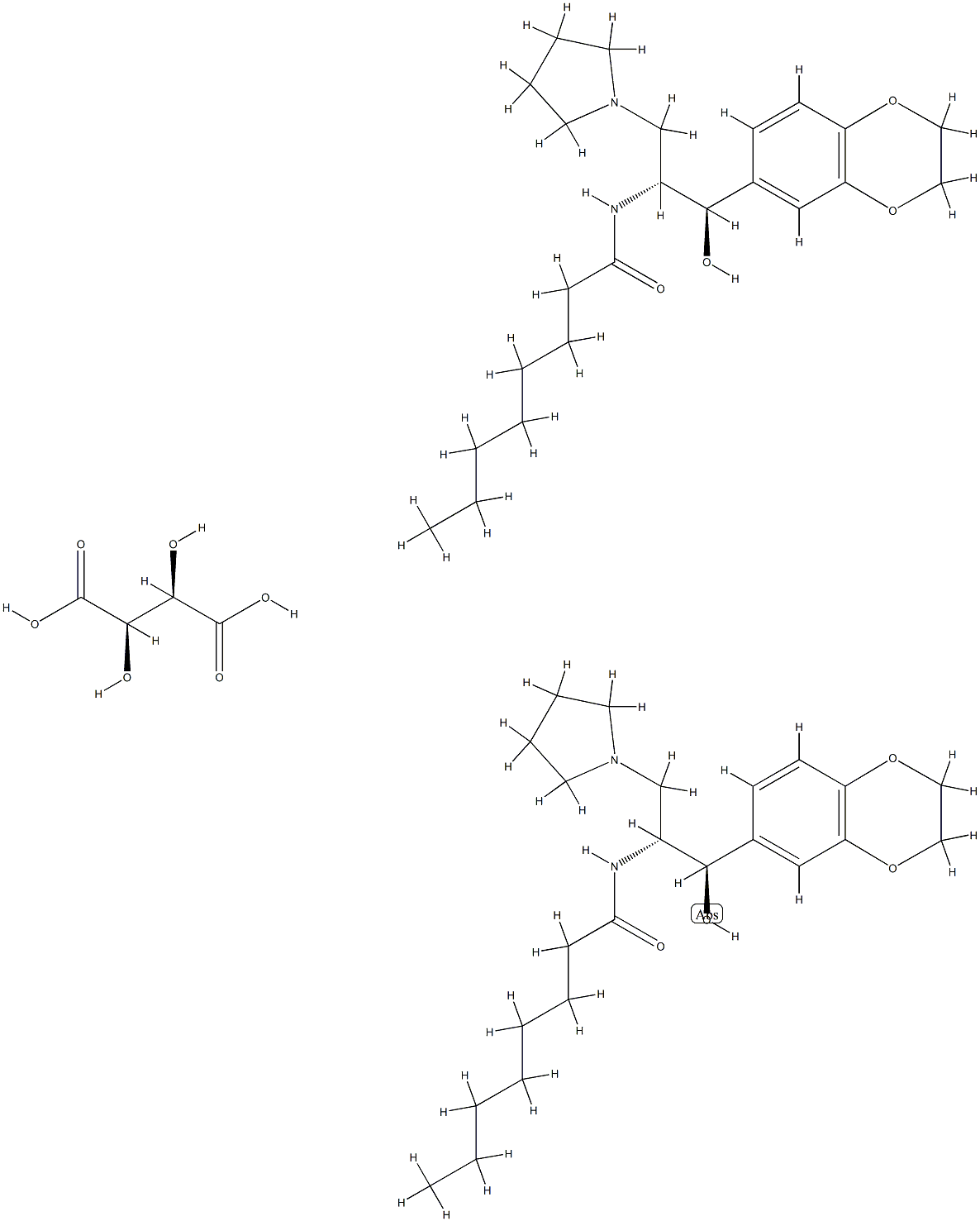
Eliglustat (Tartrate)
- CAS No.
- 928659-70-5
- Chemical Name:
- Eliglustat (Tartrate)
- Synonyms
- GENZ112638;Eligluhemitartrate;Eliglustat (Tartrate);Eliglustat (hemitartrate);Eliglustat tartrate (Genz-112638);GENZ-112638;GENZ 112638;GENZ112638;Eliglustat hemitartrate(Genz-99067);Eliglustat hemitartrate (Genz-112638);Eliglustat (Tartrate),Eliglustat tartrate;Genz112638,Inhibitor,Eliglustat,Genz 112638,inhibit,Eliglustat hemitartrate
- CBNumber:
- CB22667944
- Molecular Formula:
- C50H78N4O14
- Molecular Weight:
- 959.17272
- MDL Number:
- MFCD22665712
- MOL File:
- 928659-70-5.mol
| Melting point | >133°C (dec.) |
|---|---|
| storage temp. | -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| color | White |
| FDA UNII | N0493335P3 |
Eliglustat (Tartrate) price More Price(11)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 21487 | Eliglustat (hemitartrate) ≥98% | 928659-70-5 | 1mg | $32 | 2024-03-01 | Buy |
| Cayman Chemical | 21487 | Eliglustat (hemitartrate) ≥98% | 928659-70-5 | 5mg | $139 | 2024-03-01 | Buy |
| Cayman Chemical | 21487 | Eliglustat (hemitartrate) ≥98% | 928659-70-5 | 10mg | $243 | 2024-03-01 | Buy |
| Cayman Chemical | 21487 | Eliglustat (hemitartrate) ≥98% | 928659-70-5 | 25mg | $533 | 2024-03-01 | Buy |
| Usbiological | 448126 | Eliglustat Tartrate | 928659-70-5 | 1mg | $305 | 2021-12-16 | Buy |
Eliglustat (Tartrate) Chemical Properties,Uses,Production
Description
Eliglustat tartrate, developed and marketed by Genzyme Corporation (a subsidiary of Sanofi), was approved by the US FDA in August 2014 for the treatment of nonneuropathic (type 1) Gaucher disease (GD1) in both treatment-naive and treatment-experienced adult patients. It is the first oral treatment to be approved for first-line use in patients with Gaucher disease type 1, which is a rare lysosomal storage disease characterized by accumulation of lipid glucosylceramide (GL-1) due to insufficient production of the enzyme glucosylceramidase. Clinical complications include hepatosplenomegaly, anemia, thrombocytopenia, and bone involvement. Eliglustat is a specific inhibitor of glucosylceramide synthase with an IC50 of 10 ng/mL and acts as substrate reduction therapy for GD1; it has demonstrated non-inferiority to enzyme replacement therapy, which is the current standard of care, in Phase III trials.
Uses
Eliglustat Tartrate functions as an oral agent for treatment of Gaucher disease type 1, and lysosomal storage disorders in human. It also inhibits UDP-glucosylceramide synthase in mice thereby preventing Gaucher disease-associated with B-cell malignancy.
Definition
ChEBI: A tartrate that is the hemitartrate salt of eliglustat. A ceramide glucosyltransferase inhibitor used (as its tartrate salt) for treatment of Gaucher's disease.
Synthesis
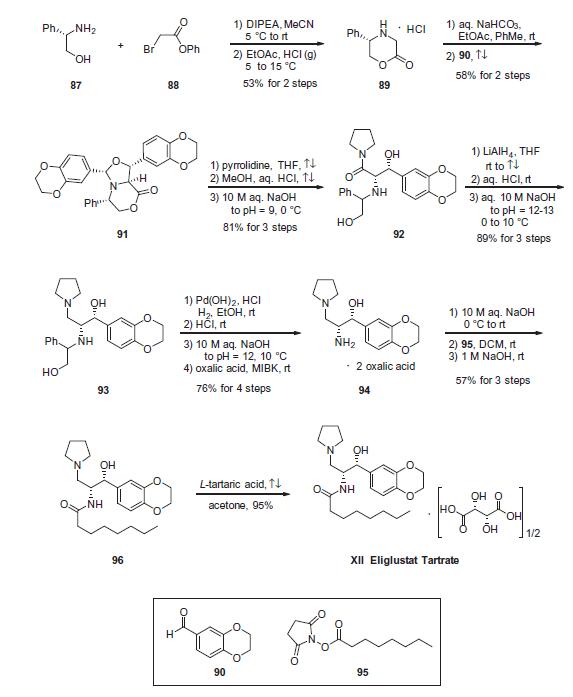
Condensation of commercially available S-(+)-2-phenyl glycinol (87) with phenyl bromoacetate (88) in acetonitrile in the presence of N,N-diisopropylethylamine (DIPEA) provided morpholin-2-one 89 upon treatment with HCl. Neutralization with NaHCO3 followed by coupling with aldehyde 90 in refluxing EtOAc/toluene yielded oxazine adduct 91, which was isolated as a precipitate from methyl-tert-butyl ether (MTBE). The stereochemistry of the three new stereocenters in 91 can be rationalized through the cycloaddition of an ylide intermediate in the sterically-preferred S-configuration (generated by the reaction of the morpholinone 89 with aldehyde 90) with a second equivalent of the aldehyde. With the morpholinone in a chair conformation in which the phenyl group is equatorial, endo axial approach of the dipolarophile to the less-hindered face of the ylide and subsequent ring flip of the morpholinone ring to a boat conformation positions all exocyclic aryl substituents in a pseudoequatorial configuration. Opening of oxazine 91 with pyrrolidine in refluxing THF followed by addition of HCl in refluxing MeOH gave amide 92, which was reduced to amine 93 using LiAlH4 in refluxing THF. Subsequent hydrogenation with Pd(OH)2 in EtOH cleaved the phenylethanol group to give the free amine, which was converted to dioxalate salt 94 by treatment with oxalic acid in methyl isobutylketone (MIBK). Subjection of aminoethanol 94 to aqueous sodium hydroxide followed by coupling with palmitic acid Nhydroxysuccinimide (NHS)-ester (95) gave eliglustat as the corresponding freebase (96) in 9.5% overall yield from 87. Salt formation with L-tartaric acid (0.5 equiv) then provided eliglustat tartrate (XII).
Eliglustat (Tartrate) Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32686 | 60 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19553 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Shanxi Xuanran Import and Export Trade Co., Ltd. | +8617735180244 | mike_yan@xuanranglobal.com | CHINA | 4022 | 58 |
| sgtlifesciences pvt ltd | +8617013299288 | dj@sgtlifesciences.com | China | 12382 | 58 |
| InvivoChem | +1-708-310-1919 +1-13798911105 | sales@invivochem.cn | United States | 6393 | 58 |
| Nantong HI-FUTURE Biology Co., Ltd. | +undefined18051384581 | sales@chemhifuture.com | China | 3136 | 58 |
| ShenZhen Trendseen Biological Technology Co.,Ltd. | 13417589054 | trendseenbio@gmail.com | China | 11681 | 58 |
| Wuhan Topule Biopharmaceutical Co., Ltd | +8618327326525 | masar@topule.com | China | 8474 | 58 |
View Lastest Price from Eliglustat (Tartrate) manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2019-12-29 | Eliglustat hemitartrate
928659-70-5
|
US $2.00 / KG | 1KG | ≥98% | 20 tons | Career Henan Chemical Co |
-

- Eliglustat hemitartrate
928659-70-5
- US $2.00 / KG
- ≥98%
- Career Henan Chemical Co