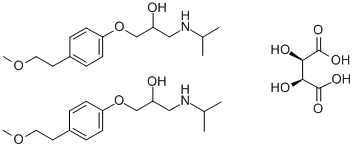
Metoprolol tartrate
- CAS No.
- 56392-17-7
- Chemical Name:
- Metoprolol tartrate
- Synonyms
- beloc;h93/26;Prelis;Niflam;betaloc;seloken;Metapro;Sipseron;lopresor;Selopral
- CBNumber:
- CB7436617
- Molecular Formula:
- 2C15H25NO3.C4H6O6
- Molecular Weight:
- 684.82
- MDL Number:
- MFCD00056257
- MOL File:
- 56392-17-7.mol
| Melting point | 120℃ |
|---|---|
| Boiling point | 695.37°C (rough estimate) |
| Density | 1.1946 (rough estimate) |
| refractive index | 1.5300 (estimate) |
| Flash point | 9℃ |
| storage temp. | 2-8°C |
| solubility | H2O: soluble50mg/mL |
| form | powder |
| color | white to off-white |
| Water Solubility | Soluble in water (>1000 mg/ml), methanol (>500 mg/ml), chloroform (496 mg/ml), ethanol (31 mg/ml at 25°C), and DMSO (100 mg/ml at 25°C). |
| λmax | 223nm(H2O)(lit.) |
| Merck | 14,6151 |
| BCS Class | 1 |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 56392-17-7(CAS DataBase Reference) |
| FDA UNII | W5S57Y3A5L |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H361d-H411 | |||||||||
| Precautionary statements | P201-P202-P273-P280-P308+P313-P391 | |||||||||
| Hazard Codes | F,T | |||||||||
| Risk Statements | 11-23/24/25-39/23/24/25 | |||||||||
| Safety Statements | 7-16-36/37-45-24/25 | |||||||||
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | UB7450100 | |||||||||
| HS Code | 29225090 | |||||||||
| Toxicity | LD50 in female mice, male rats (mg/kg): 118, ~90 i.v.; 2090, 3090 orally (Bodin) | |||||||||
| NFPA 704 |
|
Metoprolol tartrate price More Price(36)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | M-123 | Metoprolol tartrate solution 1.0?mg/mL in methanol (as free base), ampule of 1?mL, certified reference material, Cerilliant? | 56392-17-7 | 1mL | $36.6 | 2024-03-01 | Buy |
| Sigma-Aldrich | BP540 | Metoprolol tartrate British Pharmacopoeia (BP) Reference Standard | 56392-17-7 | 100MG | $233 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1441301 | Metoprolol tartrate United States Pharmacopeia (USP) Reference Standard | 56392-17-7 | 200mg | $436 | 2024-03-01 | Buy |
| Sigma-Aldrich | 80337 | (±)-Metoprolol (+)-tartrate salt analytical standard | 56392-17-7 | 100mg | $58.6 | 2022-05-15 | Buy |
| Alfa Aesar | J61920 | Metoprolol tartrate, 98+% | 56392-17-7 | 5g | $78.5 | 2024-03-01 | Buy |
Metoprolol tartrate Chemical Properties,Uses,Production
β1 receptor blocker
Metoprolol tartrate is a selective β1-adrenergic receptor blocker,oral absorption is rapid and complete,it is used for the treatment of hypertension, angina pectoris, myocardial infarction, hypertrophic cardiomyopathy, aortic dissection, cardiac arrhythmia, hyperthyroidism, cardiac neurosis and other diseases. In recent years, it is also used for ther treatment of heart failure, which can reduce mortality. In unstable/non-ST-segment elevation myocardial infarction and acute myocardial infarction control guiding principles developed by American Heart Association , effect of metoprolol tartrateare is clearly recognized. In the past, concerns about β-blocker therapy caused hypotension is too large, and for the consideration that there may be racial differences between Asians in β-blocker use and the West, patients with treatment of β-blockers in China is fewer , the use of β-blockers is in lower dose.
Metoprolol tartrate has a weaker membrane stability and it has no intrinsic sympathomimetic activity, and in comparison with similar drugs commonly used in clinical propranolol ,in addition to the advantages of β1-blocker on its pharmacodynamic,it also has its pharmacokinetic kinetics advantages, such as relatively weak first-pass effect compared with propranolol, simple metabolic pathways, most pharmacologically inactive metabolites and the like.
Uses
Hypertensive medication.
Hazards & Safety Information
Category: toxic substances
Toxicity grading: Middle poisoning
Acute toxicity: oral -rat LD50: 5500 mg/kg; Oral-Mouse LD50: 1500 mg/kg
Flammability and hazard characteristics: It is combustible; fire decomposition produces toxic nitrogen oxide gases
Storage Characteristics: Ventilated, low-temperature ,dry storeroom.
Extinguishing agent: Water, carbon dioxide, dry powder, sandy soil.
Description
Metoprolol (tartrate) (Item No. 21165) is an analytical reference standard categorized as a β1-adrenergic receptor blocker. This product is intended for use in analytical forensic applications. Metoprolol (succinate) is available as a general research tool .
Chemical Properties
White or almost white, crystalline powder or colourless crystals.
Originator
Betaloc,Astra,UK,1975
Uses
A b1-Adrenergic blocker
Uses
alpha(1)-adrenergic blocker
Uses
A ? selective aryloxypropanolamine andrenergic antagonist. Used in the treatment of a variety of cardiovascular disorder. Antihypertensive; antianginal; antiarrhythmic (class II)
Uses
A β1 selective aryloxypropanolamine andrenergic antagonist. Used in the treatment of a variety of cardiovascular disorder. Antihypertensive; antianginal; antiarrhythmic (class II).
Uses
Metoprolol tartrate is an antagonist of the β1-AR (β1-adrenoceptor). It inhibits spontaneous endothelin-1 production in vitro and displays antihypertensive and antianginal activity in vivo.
Manufacturing Process
The starting material 1,2-epoxy-3-[p-(β-methoxyethyl)phenoxy]-propane was
obtained from p-(β-methoxyethyl)-phenol which was reacted with
epichlorohydrin whereafter the reaction product was distilled at 118°C to
128°C at a pressure of 0.35mm Hg.
1,2-Epoxy-3-[p-(β-methoxyethyl)-phenoxy]-propane (16.7g) was dissolved in
50 ml isopropanol and mixed with 20 ml isopropylamine. The mixture was
heated in an autoclave on boiling water-bath overnight, whereafter it was
evaporated and the remainder dissolved in 2 N HCI. The solution was
extracted first with ether and thereafter with methylene chloride. After
evaporating the methylene chloride phase, the hydrochloride of 1-
isopropylamino-3-[p(β-methoxyethyl)-phenoxy] -propanol-2 was obtained
which, after recrystallization from ethyl acetate, weighed 10.4 g. Melting point
83°C. Equivalent weight: found 304.0, calculated 303.8.
The hydrochloride is then converted to the tartrate.
brand name
Lopressor (Novartis).
Therapeutic Function
Beta-adrenergic blocker
General Description
A certified solution standard applicable for use in clinical toxicology or forensic analysis by LC-MS/MS or GC/MS. Metoprolol is used for the treatment of multiple heart conditions including hypertension, angina, and tachycardia. This beta blocker is sold under the trade names Lepressor and Toprol XL?.
Biological Activity
Selective β 1 -adrenoceptor antagonist (K i values are 47, 2960 and 10100 nM for β 1 , β 2 and β 3 adrenoceptors respectively). Inhibits spontaneous endothelin-1 production in vitro and displays antihypertensive and antianginal activity in vivo .
Clinical Use
Beta-adrenoceptor blocker:
Hypertension
Angina
Cardiac arrhythmias
Migraine prophylaxis
Hyperthyroidism
Veterinary Drugs and Treatments
Because metoprolol is relatively safe to use in animals with bronchospastic disease, it is often chosen over propranolol. It may be effective in supraventricular tachyarrhythmias, premature ventricular contractions (PVC’s, VPC’s), systemic hypertension, and treating cats with hypertrophic cardiomyopathy. There is increasing interest in using beta blockers in heart failure in dogs; one retrospective study showed increased survival times when dogs were given metoprolol, but definitive prospective, double-blinded studies have not been reported documenting the benefit (increased survival) of beta-blockers in dogs with heart failure.
Drug interactions
Potentially hazardous interactions with other drugs
Anaesthetics: enhanced hypotensive effect.
Analgesics: NSAIDs antagonise hypotensive effect.
Anti-arrhythmics: increased risk of myocardial
depression and bradycardia; increased risk of
bradycardia, myocardial depression and AV block
with amiodarone; increased risk of myocardial
depression and bradycardia with flecainide;
concentration increased by propafenone and
dronedarone.
Antibacterials: concentration reduced by rifampicin.
Antidepressants: enhanced hypotensive effect with
MAOIs; concentration increased by citalopram and
escitalopram and possibly by paroxetine - avoid.
Antihypertensives; enhanced hypotensive effect;
increased risk of withdrawal hypertension with
clonidine; increased risk of first dose hypotensive
effect with post-synaptic alpha-blockers such as
prazosin.
Antimalarials: increased risk of bradycardia with
mefloquine; avoid with artemether/lumefantrine.
Antipsychotics enhanced hypotensive effect with
phenothiazines.
Antivirals: avoid concomitant use with tipranavir in
heart failure.
Calcium-channel blockers: increased risk of
bradycardia and AV block with diltiazem;
hypotension and heart failure possible with
nifedipine and nisoldipine; asystole, severe
hypotension and heart failure with verapamil.
Cytotoxics: possible increased risk of bradycardia
with crizotinib.
Diuretics: enhanced hypotensive effect.
Fingolimod: possibly increased risk of bradycardia.
Moxisylyte: possible severe postural hypotension.
Metabolism
Extensively metabolised in the liver, mainly by the cytochrome P450 isoenzyme CYP2D6, and undergoes oxidative deamination, O-dealkylation followed by oxidation, and aliphatic hydroxylation. The metabolites are excreted in the urine with only small amounts of unchanged metoprolol.
storage
Desiccate at RT
Metoprolol tartrate Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15928 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Xi'an Kono chem co., Ltd., | 029-86107037 13289246953 | info@konochemical.com | China | 2995 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Alchem Pharmtech,Inc. | 8485655694 | sales@alchempharmtech.com | United States | 63711 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49390 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
View Lastest Price from Metoprolol tartrate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-06-26 | Metoprolol Tartrate
56392-17-7
|
US $200.00 / kg | 1kg | 99% | 1000kg/Month | Hebei Mingeng Biotechnology Co., Ltd | |
 |
2023-04-11 | Metoprolol Tartrate
56392-17-7
|
US $100.00 / mg | 25mg | 99% | 1000g | Shijiazhuang Gantuo Biotechnology Co., Ltd | |
 |
2023-02-09 | Metoprolol tartrate
56392-17-7
|
US $0.00 / KG | 1KG | 98%min | 30tons/month | WUHAN FORTUNA CHEMICAL CO., LTD |
-

- Metoprolol Tartrate
56392-17-7
- US $200.00 / kg
- 99%
- Hebei Mingeng Biotechnology Co., Ltd
-

- Metoprolol Tartrate
56392-17-7
- US $100.00 / mg
- 99%
- Shijiazhuang Gantuo Biotechnology Co., Ltd
-

- Metoprolol tartrate
56392-17-7
- US $0.00 / KG
- 98%min
- WUHAN FORTUNA CHEMICAL CO., LTD