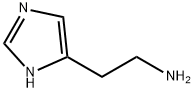
Histamine
- CAS No.
- 51-45-6
- Chemical Name:
- Histamine
- Synonyms
- 2-(1H-Imidazol-4-yl)ethan-1-amine;2-(1H-Imidazol-4-yl)ethanamine;HISTAMIN;Eramin;Eramine;Ergamine;istamina;HIS ELISA;Theramine;HISTAMINE
- CBNumber:
- CB3326778
- Molecular Formula:
- C5H9N3
- Molecular Weight:
- 111.15
- MDL Number:
- MFCD00005210
- MOL File:
- 51-45-6.mol
- MSDS File:
- SDS
| Melting point | 83-84 °C (lit.) |
|---|---|
| Boiling point | 167 °C/0.8 mmHg (lit.) |
| Density | 0.9902 (rough estimate) |
| refractive index | 1.4690 (estimate) |
| storage temp. | -20°C |
| solubility | DMSO: 20 mg/ml; Ethanol: 10 mg/ml; PBS (pH 7.2): 10 mg/ml |
| pka | 6.04(at 25℃) |
| form | Solid |
| color | White to light yellow |
| Water Solubility | Soluble in water, alcohol, and hot chloroform. |
| Merck | 13,4739 |
| BRN | 2012 |
| Stability | Hygroscopic |
| InChIKey | NTYJJOPFIAHURM-UHFFFAOYSA-N |
| LogP | -0.700 |
| CAS DataBase Reference | 51-45-6(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| NCI Dictionary of Cancer Terms | histamine |
| FDA UNII | 820484N8I3 |
| NIST Chemistry Reference | Histamine(51-45-6) |
| EPA Substance Registry System | Histamine (51-45-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301-H315-H317-H319-H334-H335 | |||||||||
| Precautionary statements | P280-P301+P310+P330-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 22-36/37/38-42/43 | |||||||||
| Safety Statements | 22-26-36/37 | |||||||||
| RIDADR | UN 2811 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | MS1050000 | |||||||||
| F | 3-10-23 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1(b) | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29332900 | |||||||||
| Toxicity | LD50 i.p. in mice: 2020 mg/kg (Nagai) | |||||||||
| NFPA 704 |
|
Histamine price More Price(26)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | H7125 | Histamine ≥97.0% | 51-45-6 | 1g | $117 | 2024-03-01 | Buy |
| Sigma-Aldrich | 59964 | Histamine analytical standard | 51-45-6 | 100mg | $71.6 | 2024-03-01 | Buy |
| Sigma-Aldrich | 3779 | Histamine, Free Base - CAS 51-45-6 - Calbiochem | 51-45-6 | 1g | $165 | 2024-03-01 | Buy |
| Sigma-Aldrich | 3779 | Histamine, Free Base - CAS 51-45-6 - Calbiochem | 51-45-6 | 5G | $454 | 2022-05-15 | Buy |
| Alfa Aesar | J61727 | Histamine | 51-45-6 | 1g | $99.8 | 2024-03-01 | Buy |
Histamine Chemical Properties,Uses,Production
Chemical Properties
White to slightly yellow powder
History
Histamine is an important protein involved in many allergic reactions. Allergies are caused by an immune response to a normally innocuous substance (i.e. pollen, dust) that comes in contact with lymphocytes specific for that substance, or antigen. The history of histamine and the development of antihistamines have been reviewed in [Drugs of Today (1986) and the Journal of Allergy & Clinical Immunology]. Histamine was the first to be characterized of a series of biogenic amines that are released in the inflammatory process. As early as 1910, it was shown that histamine caused constriction of isolated guinea pig ileum and, subsequently, it was found that histamine induced a shock-like syndrome. In 1927 the presence of histamine in normal tissues was demonstrated. Attempts to reduce histamine manifestations led to the report, in 1933, that certain phenolic ethers inhibited histamine action. Toxicity precluded clinical use. In 1942 phenbenzamine (Antergan), C17H22N2, was the first antihistamine to be successfully used in humans.
In 1966, the name H1 was proposed for receptors blocked by the at that time known antihistamines. It was also speculated that the other actions of histamine were likely to be mediated by other histamine receptors. The existence of the H2 receptor was accepted in 1972 and the H3 receptor was recognized in rat brain in 1983. H3 receptors in the brain appear to be involved in the feedback control of both histamine synthesis and release, whereas release of various other neurotransmitters, eg, serotinin (5-HT), dopamine, noradrenaline, and acetylcholine, is also modulated. H3 receptor effects have also been demonstrated in various peripheral tissues and H3 agonists and antagonists are undergoing intensive study for therapeutic applications.
Uses
H1&2 agonist, edema induction, gastric secretion stimulant
Uses
Histamine inhibits the synthesis of IL-2 and γ-IFN in peripheral blood mononuclear cells and lipopolysaccharide-induced synthesis of TNF-α in monocytes via H2?receptor activation. It is a powerful stimulant of gastric secretion, a constrictor of bronchial smooth muscle, a vasodilator, and also a centrally acting neurotransmitter.
Indications
Sinus problems, hay fever, bronchial asthma, hives, eczema, contact dermatitis, food allergies, and reactions to drugs are all allergic reactions associated with the release of histamine and other autocoids, such as serotonin, leukotrienes, and prostaglandins. Histamine release is frequently associated with various inflammatory states and may be increased in urticarial reactions, mastocytosis, and basophilia. Histamine also acts as a neurotransmitter in the central nervous system (CNS). Upon release from its storage sites, histamine exerts effects ranging from mild irritation and itching to anaphylactic shock and eventual death.
Definition
ChEBI: A member of the class of imidazoles that is 1H-imidazole substituted at position C-4 by a 2-aminoethyl group.
Biosynthesis
Virtually all of the histamine found in individual organs
and tissues is synthesized locally and stored in subcellular
secretory granules. Within the tissues, the mast cells
are the principal sites of storage; in the blood, the basophils serve this function. Histamine is also present in
neurons of the CNS, where it acts as a neurotransmitter.
Histamine is synthesized from the amino acid histidine
by an action of the enzyme histidine decarboxylase. Following synthesis, histamine is either rapidly
inactivated or stored in the secretory granules of
mast cells and basophils as an inactive complex with
proteases and heparin sulfate or chondroitin sulfate.
Biological Functions
Histamine occurs in the brain, particularly in certain hypothalamic neurons, and evidence is strong that histamine is a neurotransmitter. Distribution of histamine, its synthetic enzyme (histidine decarboxylase), and methyl histamine (the major brain metabolite) is not uniform. Possible roles for histamine in the regulation of food and water intake, thermoregulation, hormone release, and sleep have been suggested.
General Description
Histamine is a neurotransmitter produced by neurons of the posterior hypothalamus. In the brain, histamine is predominantly present in the gray matter.
Biochem/physiol Actions
Histamine participates in innate and acquired immune response, mediating allergy and inflammation. It helps in intestinal muscle contraction. During anaphylactic shock histamine causes bronchial constriction. Histamine is also involved in gastric acid secretion, epithelial and endothelial barrier control.
Mechanism of action
Non–Antigen-Mediated Release of Histamine Histamine may be released from mast cells by mechanisms that do not require prior sensitization of the immune system. Drugs, high-molecular-weight proteins, venoms, and other substances that damage or disrupt cell membranes can induce the release of histamine. Any thermal or mechanical stress of sufficient intensity also will result in histamine release. Cytotoxic compounds, may release histamine as the result of disruption of cell membranes.
Pharmacology
Histamine is found in animal tissues and venoms and in many bacteria and plants.Within the human body, the largest histamine concentrations are in the skin, lungs, and gastrointestinal mucosa, while concentrations are smaller in almost all other organs and tissues.Histamine is present in human plasma at relatively low concentrations (usually less than 0.5 ng/mL); in contrast, wholeblood levels can be as high as 30-fold greater. Substantial quantities of histamine are present in urine, with excretion rates varying from 10 to 40μg per 24 hours.
Clinical Use
Histamine has only minor uses in clinical medicine. In the past it was used to diagnose pernicious anemia, in which histamine fails to evoke the usual secretion of gastric acid. Histamine has been used to assess bronchial hyperreactivity, although this test may be quite hazardous for asthmatics. Today the main clinical use of histamine is as a positive control injection for allergy skin testing.
Side effects
Sedation is the most frequent adverse reaction to the
first-generation antihistamines. An additive effect on
alertness and motor skills will result if alcohol or another
depressant is taken with these drugs. Antimuscarinic
effects caused by these drugs include dry
mouth and respiratory passages, urinary retention, and
dysuria. Nausea, vomiting, constipation or diarrhea,
dizziness, insomnia, nervousness, and fatigue also have
been reported. Drug allergy, especially after topical application,
is fairly common.Tolerance to certain antihistamines
may develop after prolonged administration.
Teratogenic effects of the piperazine antihistamines
have been shown in animal studies. Epidemiological studies have not shown such an association in humans.
The effects of toxic doses of first-generation antihistamines,
similar to those seen following atropine administration,
include excitement, hallucinations, dry mouth,
dilated pupils, flushing, convulsions, urinary retention,
sinus tachycardia, coma, and death.
The second-generation H1-antagonists are often referred
to as nonsedating antihistamines; however, doses
above the usual therapeutic level can cause sleepiness
in certain individuals.A more serious adverse effect of
some earlier second-generation antihistamines is cardiotoxicity.
Synthesis
Histamine is synthesized in tissues by decarboxylation of amino acid L-histidine, a process catalyzed by the pyridoxalphosphate-dependent enzyme L-histidinedecarboxylase. Histamine can enter the organism with food; it also can be generated by bacteria of the gastrointestinal tract.
Purification Methods
It crystallises from *benzene or chloroform. [Beilstein 25 I 628, 25 II 302, 25 III/IV 2049.]
Histamine Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49390 | 58 |
| Zhengzhou Alfa Chemical Co.,Ltd | +8618530059196 | sale04@alfachem.cn | China | 12527 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| ANHUI WITOP BIOTECH CO., LTD | +8615255079626 | eric@witopchemical.com | China | 23556 | 58 |
Related articles
- synthesis and Use of Histamine
- Histamine is produced by basophils and mast cells and is involved in inflammatory responses.
- Jun 23,2022
View Lastest Price from Histamine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2021-09-29 | Histamine
51-45-6
|
US $0.00 / g | 1g | 98.0%min BR | 1000G | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2021-08-13 | Histamine USP/EP/BP
51-45-6
|
US $1.10 / g | 1g | 0.999 | 100 Tons min | Dideu Industries Group Limited | |
 |
2021-07-13 | Histamine
51-45-6
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd |
-

- Histamine
51-45-6
- US $0.00 / g
- 98.0%min BR
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Histamine USP/EP/BP
51-45-6
- US $1.10 / g
- 0.999
- Dideu Industries Group Limited
-

- Histamine
51-45-6
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd