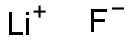Lithium fluoride
- CAS No.
- 7789-24-4
- Chemical Name:
- Lithium fluoride
- Synonyms
- Lithium fluoride (LiF);LITHIUM FLUOIRDE;Lithium Flouride;lithium fluoride, puratronic;ntl50;NTL 50;tld100;tld700;TLD 100;TLD 700
- CBNumber:
- CB5854343
- Molecular Formula:
- FLi
- Molecular Weight:
- 25.94
- MDL Number:
- MFCD00011090
- MOL File:
- 7789-24-4.mol
- MSDS File:
- SDS
| Melting point | 845 °C (lit.) |
|---|---|
| Boiling point | 1681 °C |
| Density | 2.64 g/mL at 25 °C (lit.) |
| vapor pressure | 0Pa at 25℃ |
| refractive index | 1.3915 |
| Flash point | 1680°C |
| storage temp. | Store at +5°C to +30°C. |
| solubility | Soluble in 0.29 g/100 mL (20°C) and hydrogen fluoride. Insoluble in alcohol. |
| form | random crystals |
| color | White to off-white |
| Specific Gravity | 2.635 |
| PH | 6.0-8.5 (25℃, 0.01M in H2O) |
| Water Solubility | 0.29 g/100 mL (20 ºC) |
| Sensitive | Hygroscopic |
| λmax |
λ: 260 nm Amax: ≤0.01 λ: 280 nm Amax: ≤0.01 |
| Merck | 14,5531 |
| Solubility Product Constant (Ksp) | pKsp: 2.74 |
| Exposure limits |
ACGIH: TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3; TWA 2.5 mg/m3 |
| Stability | Stable, but hygroscopic. Hydrolyzes in the presence of water to form hydrofluoric acid, which attacks glass - do not store in glass bottles. Incompatible with aqueous solutions, strong acids, strong oxidizing agents. |
| InChIKey | PQXKHYXIUOZZFA-UHFFFAOYSA-M |
| LogP | 0.23 at 25℃ |
| CAS DataBase Reference | 7789-24-4(CAS DataBase Reference) |
| EWG's Food Scores | 4 |
| FDA UNII | 1485XST65B |
| NIST Chemistry Reference | Lithium fluoride(7789-24-4) |
| EPA Substance Registry System | Lithium fluoride (7789-24-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301-H315-H319-H335 | |||||||||
| Precautionary statements | P301+P310+P330-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | T | |||||||||
| Risk Statements | 25-32-36/37/38-23/24/25 | |||||||||
| Safety Statements | 22-26-36/37/39-45 | |||||||||
| RIDADR | UN 3288 6.1/PG 3 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | OJ6125000 | |||||||||
| F | 10-21 | |||||||||
| Hazard Note | Toxic | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 28261900 | |||||||||
| Toxicity | LD in guinea pigs (mg/kg): 200 orally, 2000 s.c. (Waldbott) | |||||||||
| NFPA 704 |
|
Lithium fluoride price More Price(75)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 932310 | Lithium fluoride | 7789-24-4 | 5G | $94.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | 203645 | Lithium fluoride powder, <100 μm, ≥99.98% trace metals basis | 7789-24-4 | 5g | $67.6 | 2024-03-01 | Buy |
| Sigma-Aldrich | 203645 | Lithium fluoride powder, <100 μm, ≥99.98% trace metals basis | 7789-24-4 | 25g | $226 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.05686 | Lithium fluoride 99.99 Suprapur? | 7789-24-4 | 50g | $462 | 2024-03-01 | Buy |
| Sigma-Aldrich | 62497 | Lithium fluoride BioUltra, ≥99.0% (F) | 7789-24-4 | 500g | $372 | 2022-05-15 | Buy |
Lithium fluoride Chemical Properties,Uses,Production
Description
Lithium Fluoride (LiF) has the lowest refractive index of all common infrared materials. It possesses the highest UV transmission of any material, being able to transmit significantly into the VUV region at the hydrogen Lyman-alpha line (121nm). Lithium fluoride can be applied in rechargeable Li batteries, in radiation dosimeter for personnel monitoring as well as radiation research, as an optical material, as a heat sink material, to produce ceramics, and to dissolve fluid fuel for molten salt reactors.

lithium fluoride crystal
Uses
Lithium Fluoride (LiF) is a water insoluble Lithium source for use in oxygen-sensitive applications, such as metal production. It is most widely used as a flux in the production of ceramics, such as enamels, glasses and glazes. Similarly it is also used in brazing and welding fluxes and molten salt chemistry in metallurgy.
Lithium fluoride is also used for:
- X-Ray monochromator plates as an analysis crystal: Lithium fluoride is also used for X-ray monochromator plates where its lattice spacing makes it the most useful analysis crystal.
- Heat sink materials
- UV transmission windows: Lithium fluoride is the material with the most extreme UV transmission of all and is used for special UV optics. Lithium fluoride transmits well into the VUV region at the hydrogen Lyman-alpha line (121nm) and beyond.
References
[1] H. Li, G. Richter, J. Maier, Reversible Formation and Decomposition of LiF Clusters Using Transition Metal Fluorides as Precursors and Their Application in Rechargeable Li Batteries, Advanced Materials, 2003, vol. 15, 736-739
[2] J. R. Cameron, F. Daniels, N. Johnson, G. Kenney, Radiation Dosimeter Utilizing the Thermoluminescence of Lithium Fluoride, Science, 1961, vol. 134, 333-334
[3] E. T. Kvamme, J. C. Earthman, D. B. Leviton, B. J. Frey, Lithium fluoride material properties as applied on the NIRCam instrument, Proc. SPIE 59,4, 2005
[4] Y. Kogure, H. Kaburaki, Y. Hiki, Low-Temperature Thermal Properties of Lithium Fluoride Containing Dislocations, Phonon Scattering in Condensed Matter, 1979, 267-270
[5] H. Naghib-zadeh, C. Glizky, I. D?rfel, T. Rabe, Low temperature sintering of barium titanate ceramics assisted by addition of lithium fluoride-containing sintering additives, Journal of the European Ceramic Society, 2010, vol. 30, 81-86
[6] M. W. Rosenthal, P. R. Kasten, R. B. Briggs, Molten-Salt Reactors – History, Status, and Potential, 1970, vol. 8, 107-117
Description
lithium fluoride is a strong irritant to the eyes and skin; potassium bromide is toxic by ingestion and inhalation; sodium chloride is table salt, a medical concern when ingested in excess, but certainly of no significant hazard to emergency responders.
Chemical Properties
Lithium fluoride is a white crystalline solid. It is not hygroscopic as are the other lithium halides and is not affected by exposure to the air. Lithium fluoride is the least soluble of the alkali metal fluorides. This characteristic likens it to the alkaline earth fluorides. Lithium fluoride is different from the other lithium halides in that it does not form hydrates which can be isolated from solution. Lithium fluoride does show an increase in solubility as hydrofluoric acid is added to an aqueous solution. Under these conditions the fluoride ion is converted to the bifluoride ion, HF-2, allowing further dissolving of the solid lithium fluoride.
Physical properties
White cubic crystals; refractive index 1.3915; density 2.635 g/cm3; melts at 845°C; vaporizes at 1,676°C; very slightly soluble in water 0.27 g/100g at 18°C; soluble in hydrofluoric acid; insoluble in alcohol.
Uses
The important uses of lithium fluoride are as flux in glasses, vitreous enamels and glazes; in soldering and welding aluminum; and its prisms in infrared spectrophotometers. The compound also is used for storing solar energy.
Uses
As flux for soldering and welding aluminum, in the manufacture of vitreous enamels and glazes. Lithium fluoride prisms are used in infrared spectrophotometers.
Uses
Lithium fluoride is widely used in many fields. It is used in the soldering progress for glass lining as a cosolvent. It finds application as an additive of aluminum electrolysis and rare earth electrolysis as a crystal in X-ray spectrometry. It is also used in specialized UV optics due to its large band gap and transparency to short wave length ultraviolet radiation. It is involved to record ionizing radiation exposure from gamma rays, beta particles and neutrons in thermoluminescent dosimeters. In nuclear reactors, lithium fluoride is mixed with beryllium fluoride to form a base solvent, which is used in molten-salt reactor experiment (MSRE). Additionally, it is useful as a coupling layer to enhance electron injection in polymer light-emitting diodes (PLED) and organic light-emitting diode (OLED).
Preparation
Lithium fluoride is prepared by treating an aqueous solution of lithium hydroxide or lithium carbonate with aqueous hydrofluoric acid: LiOH + HF → LiF + H2O.
Flammability and Explosibility
Non flammable
Safety Profile
Poison by ingestion and subcutaneous routes. When heated to decomposition it emits toxic fumes of F-. Used as a flux in enamels, glasses, glazes, and weldmg. See also FLUORIDES and LITHIUM COMPOUNDS.
Purification Methods
Possible impurities are LiCO3, H2O and HF. These can be removed by calcining it at red heat, then pulverizing it with a Pt pestle and storing it in a paraffin bottle. Its solubility in H2O is 0.27% at 18o. It volatilises between 1100-1200o. [Kwasnik in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 235 1963].
Structure and conformation
The space lattice of LiF belongs to the cubic system, and its rock salt structure has a lattice constant of a=0.40173 nm and Li-F=0.201 nm. The cleavage plane is (100).
Lithium fluoride Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shanghai Huaran Industrial Co.,Ltd | 17749774353 | 1715438216@qq.com | China | 52 | 58 |
| Frapp's ChemicalNFTZ Co., Ltd. | +86 (576) 8169-6106 | sales@frappschem.com | China | 885 | 50 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| QINGDAO HONG JIN CHEMCIAL CO.,LTD. | 532-83657313 | hjt@hong-jin.com | China | 228 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8823 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49390 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
View Lastest Price from Lithium fluoride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-24 | LiF
7789-24-4
|
US $0.00 / g | 1g | 99.99% | 100KGS | Zhejiang Free Trade Zone Violet Trade Co., Ltd. | |
 |
2023-07-25 | Lithium Fluoride
7789-24-4
|
US $50.00 / Kg/Bag | 25KG | 99%;99.95% | 800tons/month | Hebei Yanxi Chemical Co., Ltd. | |
 |
2023-01-31 | Lithium fluoride
7789-24-4
|
US $27.00 / KG | 1KG | 99% | 20tons | Hebei Mojin Biotechnology Co., Ltd |
-
- LiF
7789-24-4
- US $0.00 / g
- 99.99%
- Zhejiang Free Trade Zone Violet Trade Co., Ltd.
-

- Lithium Fluoride
7789-24-4
- US $50.00 / Kg/Bag
- 99%;99.95%
- Hebei Yanxi Chemical Co., Ltd.
-

- Lithium fluoride
7789-24-4
- US $27.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
7789-24-4(Lithium fluoride)Related Search:
1of4