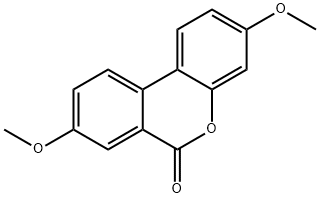
Urolithin A
- CAS No.
- 1143-70-0
- Chemical Name:
- Urolithin A
- Synonyms
- 3,8-dihydroxybenzo[c]chromen-6-one;NeolithicA;Urolithin A;urolithin-A(UA;JACS-1143-70-0;Urolithin A >97%;Urinary calculus a;Castoreum pigment I;3,8-Dihydroxyurolithin;CaChemicalbookstoreumpigmentI
- CBNumber:
- CB61350281
- Molecular Formula:
- C13H8O4
- Molecular Weight:
- 228.2
- MDL Number:
- MFCD20275235
- MOL File:
- 1143-70-0.mol
- MSDS File:
- SDS
| Melting point | 340-345 °C |
|---|---|
| Boiling point | 527.9±43.0 °C(Predicted) |
| Density | 1.516±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO (Slightly), Methanol (Very Slightly) |
| form | powder |
| pka | 9.07±0.20(Predicted) |
| form | Solid/Powder |
| color | white to beige |
| color | Beige to Yellow |
| InChI | InChI=1S/C13H8O4/c14-7-1-3-9-10-4-2-8(15)6-12(10)17-13(16)11(9)5-7/h1-6,14-15H |
| InChIKey | RIUPLDUFZCXCHM-UHFFFAOYSA-N |
| SMILES | C12=CC(O)=CC=C1C1=CC=C(O)C=C1C(=O)O2 |
| LogP | 2.311 (est) |
| FDA UNII | ILJ8NEF6DT |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319 | |||||||||
| Precautionary statements | P305+P351+P338 | |||||||||
| NFPA 704 |
|
Urolithin A price More Price(24)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SML1791 | Urolithin A ≥97% (HPLC) | 1143-70-0 | 5MG | $107 | 2023-06-20 | Buy |
| Sigma-Aldrich | SML1791 | Urolithin A ≥97% (HPLC) | 1143-70-0 | 25MG | $434 | 2023-06-20 | Buy |
| Cayman Chemical | 22607 | Urolithin A ≥98% | 1143-70-0 | 1mg | $34 | 2024-03-01 | Buy |
| Cayman Chemical | 22607 | Urolithin A ≥98% | 1143-70-0 | 5mg | $60 | 2024-03-01 | Buy |
| Cayman Chemical | 22607 | Urolithin A ≥98% | 1143-70-0 | 10mg | $85 | 2024-03-01 | Buy |
Urolithin A Chemical Properties,Uses,Production
Description
Urolithin A is a metabolite of ellagic acid. It is produced by the body after you ingest compounds found in particularly high concentration in pomegranates (particularly the bitter components such as the skin and seeds) and can help recycle defective mitochondria. Since it is a metabolite that results from the transformation of the tannins in pomegranate by gut bacteria, it can be classified as a postbiotic.It has been demonstrated to stimulate mitophagy and improve muscle health in old animals and in preclinical models of aging.
Uses
Urolithin A(UA) is a major metabolite of ellagitannin and exhibits anti-inflammatory and antioxidant properties. UA also has the ability to induce autophagy and apoptosis, inhibit cell cycle progression, and inhibit DNA synthesis. At the physiological level, UA improved muscle function in nematodes, young rodents, old mice, and muscle-wasting disorders such as Duchenne muscle dystrophy (DMD). Long-term intake of UA improves muscle endurance. In vitro studies, it has also been shown to inhibit the growth of human cancer cells in the colon, prostate and breast.
Definition
ChEBI: Urolithin A is a member of coumarins. It has a role as a geroprotector.
Preparation
2-bromo-5-methoxybenzoic acid is coupled with resorcinol to yield the intermediate “methoxy” product (Intermediate A), which is then isolated via filtration and dried for use in step 2. The solvent methanol is utilized in this step.
the intermediate product, "Intermediate A", is treated with the Lewis acid, AlCl3, in order to obtain a crude urolithin A. The ether cleavage of Intermediate A is accomplished by activation of the methyl ether through the addition of the Lewis acid, AlCl3, in toluene. The activated species is then hydrolyzed by the addition of water. Following hydrolysis, the crude product is filtered and dried.
crude urolithin A is dissolved in dimethyl sulfoxide (DMSO) for a polish filtration and subsequently precipitated from this DMSO solution by the addition of water. The filter cake is rinsed by water, followed by methanol. The raw urolithin A is then triturated with acetic acid (HOAc) to further purify the product and then collected by filtration. Following filtration, the purified product is rinsed with HOAc followed by tert-butyl-methyl ether (TBME), then dried to yield the final product, urolithin A.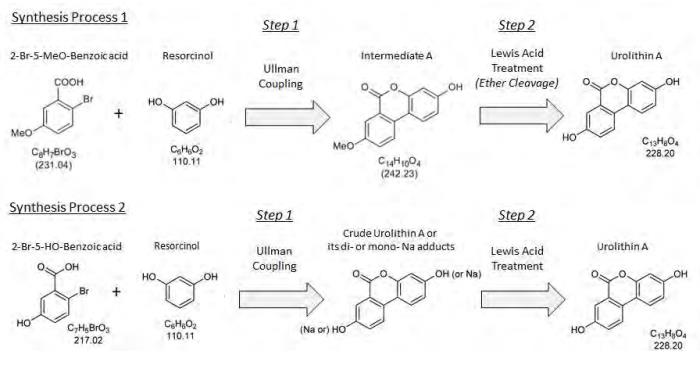
Synthesis of Urolithin A
benefits
As you age, ATP production begins to put strain on your mitochondria, and eventually, energy output falls. But when exposed to urolithin A, these failing mitochondria are broken down and eliminated (very similar to taking out the trash!) to make room for new, properly functioning mitochondria to grow.
Ellagitannins and punicalagins are two natural polyphenols found in pomegranates. They have been shown to have anti-inflammatory and anticancer effects, but once metabolized by gut bacteria, they also produce urolithin A in the digestive tract. So supplementation with pomegranate extract, along with specific bacterial species (probiotics) that can help the pomegranate compounds to produce urolithin A, can be an effective approach to maintaining healthy mitochondria.
Biochem/physiol Actions
Urolithin A is a natural product with antiproliferative and antioxidant activity. Urolithin A is formed by metabolism from polyphenols found in some nuts and fruits, particularly pomegranates. Urolithin A has been shown to cross the blood brain barrier, and may have neuroprotective effects against Alzheimer′s Disease.
Side effects
The urolithin A supplementation was generally well-tolerated, although there were a few side effects recorded: increased lipids, triglycerides, LDL cholesterol, and chest pains.(But there was very little difference between the placebo group — where similar adverse effects were recorded — and the intervention group.) Most of these were mild and only one person withdrew from the trial due to side effects.
Safety
In vivo studies did not determine any toxicity or specific adverse effects following dietary intake of urolithin A. Safety studies in elderly humans indicated urolithin A was well tolerated. In 2018, the US Food and Drug Administration listed urolithin A as a safe ingredient for food or dietary supplement products having content in the range of 250 mg to one gram per serving. The battery of genotoxicity assays demonstrated that Urolithin A (UA) is not genotoxic. The ADME study showed that glucuronidated and sulfonated forms of UA are the predominant metabolites following both oral and i.v. administration. The 28-day (0, 0.175, 1.75, and 5.0% UA mixed in diet) and 90-day studies (0, 1.25, 2.5, and 5.0% UA mixed in diet) showed no alterations in clinical parameters, blood chemistry, or hematology, and did not indicate any target organs, or any specific toxic mechanisms. The NOAEL was the highest dose tested, 5% UA by weight in the diet, or 3451 mg/kg bw/day in males and 3826 mg/kg bw/day in females in the 90-day oral study[1].
Source
Urolithin A is a natural compound found in certain foods such as pomegranates, strawberries, raspberries, and nuts such as walnuts and almonds.
Metabolism
lt appears that the majority of ingested ellagitannins and EA are metabolized by thegut microbiota into a variety of urolithins. Urolithins are dibenzopyran-6-one derivatives that are produced from EA through the loss of one of the two lactonespresent in EA and then by successive removal of hydroxyl groups.Urolithin D isproduced first,followed sequentially by urolithin C, urolithin A, and urolithin B.Urolithins appear in the circulatory system almost exclusively as glucuronide,sulfateand methylated forms as a result of phase Il metabolism after absorption in the colonand passage through the liver.
storage
Store at -20°C
Clinical claims and research
Some of the earliest research on urolithin A was performed on C. elegans worms, which experienced a 45 percent longer life span than worms not given the compound. Rodents given urolithin A experienced markedly improved muscular function and clearance of damaged mitochondria. Compared to a control group, these rodents showed a 57 to 65 percent increase in exercise capacity, 42 percent increase in running endurance, and a 9 percent increase in grip strength—all markers that correlate with longevity.
In addition, researchers have exposed colon cancer stem cells to a mixture containing urolithin A and found it to be effective at inhibiting the number and size of colon cancer stem cells and also in inhibiting the activity of aldehyde dehydrogenase, a marker of resistance to chemotherapy. Urolithin A can also cross the blood-brain barrier to protect against neurotoxicity and amyloid plaque accumulation.
References
[1] Heilman J, et al. Safety assessment of Urolithin A, a metabolite produced by the human gut microbiota upon dietary intake of plant derived ellagitannins and ellagic acid. Food and Chemical Toxicology, 2017; 108: 289-297.
Urolithin A Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Wuhan Nutra Biotechnology Co.,Ltd | +8617786394783 | nutrabiotech@outlook.com | China | 300 | 58 |
| Guangzhou TongYi biochemistry technology Co.,LTD | +8613073028829 | mack@tongyon.com | China | 2996 | 58 |
| Wuhan senwayer century chemical Co.,Ltd | +undefined-27-86652399 +undefined13627115097 | market02@senwayer.com | China | 875 | 58 |
| Maintain Biotech Co., Ltd. | +86-75589664337 +86-15112661142 | cami@maintain-bio.com | China | 66 | 58 |
| Bonerge(Hunan) Lifescience Co., Ltd. | +86-731-82791134 +86-18801900056 | hank.h@bonerge.com | China | 30 | 58 |
| Tianjin Kilo Pharmaceutical Sci-Tech Co., Ltd | +86-02223869539 +86-15560057295 | trade.kilopharma@foxmail.com | China | 27 | 58 |
| Suzhou Yuanrui Bio-Tech Co., Ltd | +86-18106132862 +86-18106132862 | sales02@yuanruibio.com | China | 278 | 58 |
| BINBO BIOLOGICAL CO.,LTD | +8618629063126 | info@binbobiological.com | China | 326 | 58 |
| Shanghai Bojing Chemical Co.,Ltd. | +86-86-02137122233 +8613795318958 | bj1@bj-chem.com | China | 298 | 55 |
| Jinan Jianfeng Chemical Co., Ltd | 0531-88110457; +8615562555968 | info@pharmachemm.com | China | 222 | 58 |
Related articles
- Urolithin A: A Natural Gut Microbiome-Derived Metabolite
- Urolithin A, derived from foods like pomegranates, shows potential in promoting health, mitigating diseases, and enhancing lon....
- Jan 2,2024
- Uses of Urolithin A in Different Diseases
- Uricetin A has various pharmacological effects such as antioxidant, anti-inflammatory, anti-atherosclerotic, anti-Aβ, anticanc....
- Nov 23,2023
- Urolithin A (UA):Sources,Chemistry properties,Health benefits
- Urolithin A (UA) is produced by gut microflora from foods rich in ellagitannins. UA has been shown to improve mitochondrial he....
- May 23,2023
View Lastest Price from Urolithin A manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-05-03 | Urolithin A
1143-70-0
|
US $0.00-0.00 / kg | 0.1kg | 99.5% | 20ton | Hangzhou Zelixir Biotech Co., Ltd. | |
 |
2024-05-02 | Urolithin A
1143-70-0
|
US $0.00 / G | 1G | 99% | 20 | CONTIDE BIOTECH CO.,LTD | |
 |
2024-04-30 | Urolithin A
1143-70-0
|
US $0.00-0.00 / KG | 1KG | 99% | 5000KG | Jinan Jianfeng Chemical Co., Ltd |
-

- Urolithin A
1143-70-0
- US $0.00-0.00 / kg
- 99.5%
- Hangzhou Zelixir Biotech Co., Ltd.
-

- Urolithin A
1143-70-0
- US $0.00 / G
- 99%
- CONTIDE BIOTECH CO.,LTD
-

- Urolithin A
1143-70-0
- US $0.00-0.00 / KG
- 99%
- Jinan Jianfeng Chemical Co., Ltd
1143-70-0(Urolithin A)Related Search:
1of4