Urolithin A Chemische Eigenschaften,Einsatz,Produktion Methoden
Beschreibung
Urolithin A is a metabolite of ellagic acid. It is produced by the body after you ingest compounds found in particularly high concentration in pomegranates (particularly the bitter components such as the skin and seeds) and can help recycle defective mitochondria. Since it is a metabolite that results from the transformation of the tannins in pomegranate by gut bacteria, it can be classified as a postbiotic.It has been demonstrated to stimulate mitophagy and improve muscle health in old animals and in preclinical models of aging.
Verwenden
Urolithin A(UA) is a major metabolite of ellagitannin and exhibits anti-inflammatory and antioxidant properties. UA also has the ability to induce autophagy and apoptosis, inhibit cell cycle progression, and inhibit DNA synthesis. At the physiological level, UA improved muscle function in nematodes, young rodents, old mice, and muscle-wasting disorders such as Duchenne muscle dystrophy (DMD). Long-term intake of UA improves muscle endurance. In vitro studies, it has also been shown to inhibit the growth of human cancer cells in the colon, prostate and breast.
Definition
ChEBI: Urolithin A is a member of coumarins. It has a role as a geroprotector.
synthetische
2-bromo-5-methoxybenzoic acid is coupled with resorcinol to yield the intermediate “methoxy” product (Intermediate A), which is then isolated via filtration and dried for use in step 2. The solvent methanol is utilized in this step.
the intermediate product, "Intermediate A", is treated with the Lewis acid, AlCl3, in order to obtain a crude urolithin A. The ether cleavage of Intermediate A is accomplished by activation of the methyl ether through the addition of the Lewis acid, AlCl3, in toluene. The activated species is then hydrolyzed by the addition of water. Following hydrolysis, the crude product is filtered and dried.
crude urolithin A is dissolved in dimethyl sulfoxide (DMSO) for a polish filtration and subsequently precipitated from this DMSO solution by the addition of water. The filter cake is rinsed by water, followed by methanol. The raw urolithin A is then triturated with acetic acid (HOAc) to further purify the product and then collected by filtration. Following filtration, the purified product is rinsed with HOAc followed by tert-butyl-methyl ether (TBME), then dried to yield the final product, urolithin A.
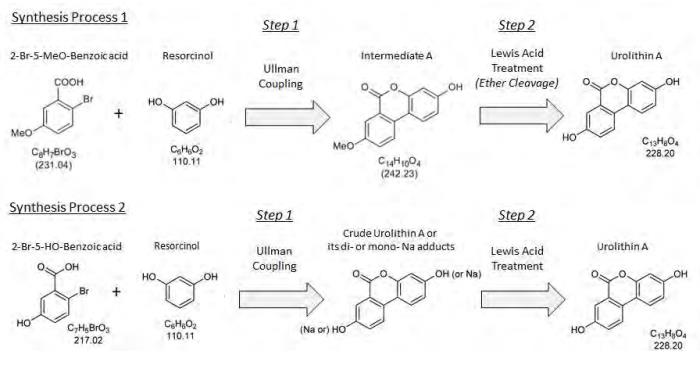
Synthesis of Urolithin A
benefits
As you age, ATP production begins to put strain on your mitochondria, and eventually, energy output falls. But when exposed to urolithin A, these failing mitochondria are broken down and eliminated (very similar to taking out the trash!) to make room for new, properly functioning mitochondria to grow.
Ellagitannins and punicalagins are two natural polyphenols found in pomegranates. They have been shown to have anti-inflammatory and anticancer effects, but once metabolized by gut bacteria, they also produce urolithin A in the digestive tract. So supplementation with pomegranate extract, along with specific bacterial species (probiotics) that can help the pomegranate compounds to produce urolithin A, can be an effective approach to maintaining healthy mitochondria.
Sicherheit(Safety)
In vivo studies did not determine any toxicity or specific adverse effects following dietary intake of urolithin A. Safety studies in elderly humans indicated urolithin A was well tolerated. In 2018, the US Food and Drug Administration listed urolithin A as a safe ingredient for food or dietary supplement products having content in the range of 250 mg to one gram per serving.
The battery of genotoxicity assays demonstrated that Urolithin A (UA) is not genotoxic. The ADME study showed that glucuronidated and sulfonated forms of UA are the predominant metabolites following both oral and i.v. administration. The 28-day (0, 0.175, 1.75, and 5.0% UA mixed in diet) and 90-day studies (0, 1.25, 2.5, and 5.0% UA mixed in diet) showed no alterations in clinical parameters, blood chemistry, or hematology, and did not indicate any target organs, or any specific toxic mechanisms. The NOAEL was the highest dose tested, 5% UA by weight in the diet, or 3451 mg/kg bw/day in males and 3826 mg/kg bw/day in females in the 90-day oral study[1].
Stoffwechsel
lt appears that the majority of ingested ellagitannins and EA are metabolized by thegut microbiota into a variety of urolithins. Urolithins are dibenzopyran-6-one derivatives that are produced from EA through the loss of one of the two lactonespresent in EA and then by successive removal of hydroxyl groups.Urolithin D isproduced first,followed sequentially by urolithin C, urolithin A, and urolithin B.Urolithins appear in the circulatory system almost exclusively as glucuronide,sulfateand methylated forms as a result of phase Il metabolism after absorption in the colonand passage through the liver.
Urolithin A Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

