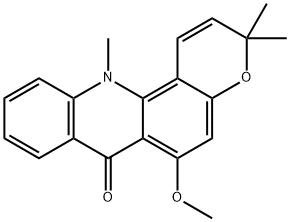
Acronine
- CAS No.
- 7008-42-6
- Chemical Name:
- Acronine
- Synonyms
- Acronine;NSC-403169;NCI-C 01536;Compound 42339;Acronycine (Acronine);3,12-Dihydro-6-methoxy-3,3,12-trimethyl-7H-pyrano[2,3-c]acridin-7-one;7H-Pyrano[2,3-c]acridin-7-one,3,12-dihydro-6-methoxy-3,3,12-trimethyl-;3,3,12-Trimethyl-6-methoxy-7,12-dihydro-3H-pyrano[2,3-c]acridine-7-one
- CBNumber:
- CB71177962
- Molecular Formula:
- C20H19NO3
- Molecular Weight:
- 321.37
- MDL Number:
- MFCD00866333
- MOL File:
- 7008-42-6.mol
- MSDS File:
- SDS
| Melting point | 175-176℃ |
|---|---|
| Boiling point | 460.13°C (rough estimate) |
| Density | 1.202±0.06 g/cm3 (20 ºC 760 Torr) |
| refractive index | 1.5600 (estimate) |
| pka | -2.43±0.40(Predicted) |
| Water Solubility | 2.7mg/L(25 ºC) |
| FDA UNII | QE0G097358 |
| EPA Substance Registry System | Acronycine (7008-42-6) |
Acronine Chemical Properties,Uses,Production
Originator
Acronine ,ZYF Pharm Chemical
Uses
Antineoplastic.
Definition
ChEBI: An alkaloid antineoplastic agent isolated from Acronychia baueri.
Manufacturing Process
The acridone alkaloids constitute a small group of natural products found
exclusively in the Rutaceae family of higher plants. A sustained interest in this
field has been due to the reported activity of acronycine a constituent of
Acronnychia baueri and Vepris amphody as an anti-tumor agent.
There are different methods of the synthetic preparation of acronycine (W. M.
Bandaranayake et al., J. Chem. Soc. Perkin 1, 998 (1968); J. Hlubucek et al.;
Aust. J. Chem. 23, 1881 (1970). One of them is described below. Friedel-
Crafts condensation between 2-nitrobenzoyl chloride and 3,5-
dimethoxyphenol.
2-Nitrobenzoyl chloride (12 g) and AlCl3, (anhyd., 13 g) were dissolved in dry
ether (50 ml) and this mixture added to a solution of 3.5-dimethoxyphenol (5
g) in dry ether 150 ml) at 0°C and the final mixture stirred at 0°C for 3
hours, brought to 20°C and stirred for a further 3 h. Diluted HCl and ice were
added and the product extracted with EtOAc (3x50 ml), this extract was
washed with aq. NaHCO3, water, dried (MgSO4), filtered and evaporated under
reduced pressure to yield a dark red oil. This oil was treated with 2 M aq.
NaOH. (100 ml) for 1 h., acidified with diluted HCl and re-extracted with EtOAc which gave, after a similar work up, a paled red oil (5.0 g). Thin layer
chromatography (TLC) showed three components one of which was the
starting phenol. Column chromatography (150 g silica gel) and elution with
benzene:petrol ether 40°-60°C (1:1) followed by increasing polarity of
solvents (benzene through chloroform to chloroform:ether (4:1) gave 59
fractions. Fractions 1-12 were combined to gave a solid, which crystallized
from benzene to give 4,6-dimethoxy-2-hydroxy-2-nitrobenzophenone, MP:
198°-199°C. Fractions 13-21 were discarded. Fractions 22-42 were combined
(0.3 g), crystallized from benzene and the product added to that obtained
from fractions 43-59 which crystallized from benzene to give 2,6-dimethoxy-
7-hydroxy-2-nitrobenzophenone (0.5 g) MP: 175°-177°C.
Condensation of 3-chloro-3-methylbutyne with 2,6-dimethoxy-7-hydroxy-2-
nitrobenzophenone:
A solution of the above benzophenone (2 g) and excess 3-chloro-3-
methylbutyne (4.5 g) in dry DMF (60 ml) containing anhydrous K2CO3 (4 g)
and dry KI (2 g) was stirred and heated at 65°C for 14 hours (under N2). The
mixture was cooled, diluted with water, acidified, extracted with chloroform
(3x50 ml) and the extract was worked up in the usual way (including a NaOH
wash) to give an oil, which was redissolved in DMF (20 ml) and heated at
130°C, under N2, for 7 h whence most of the starting material had
disappeared. The solvent was removed under reduced pressure to give a
product (0.62 g) which was purified by preparative layer chromatography on
silica gel to give 6-(2-nitrobehzoyl)-5,7-dimethoxy-2,2-dimethylchromene
(0.22 g) which crystallized from EtOH, MP: 92°-93°C.
6-(2-Aminobenzoyl)-5,7-dimethoxy-2,2-dimethylchromene (0.2 g) was
dissolved in EtOH (30 ml) containing water (5 ml) and ammonium chloride (1
g) and Zn mossy (1.5 g) was added in portions and the mixture stirred at
room temperature for 5 days. The solution was filtered, evaporated to dryness
under reduced pressure and the residue dissolved in EtOAc (25 ml) and
worked up in the usual way to give a solid (0.19 g). It crystallised from EtOH
with MP: 123°-126°C.
Cyclization of aminodimethylchromenylbenzophenone: 6-(2-aminobenzoyl)-
5,7-dimethoxy-2,2-dimethylchromene (0.12 g) was dissolved in DMSO (8 ml)
and NaH (0.06 g) added, the mixture was stirred for 6 days at room
temperature. A further addition of NaH (0.06 g) was made and the solution
heated to 50°C for 0.5 h whence it was poured into water, extracted with
EtOAc and worked up in the usual way to give a crude mixture (0.11 g;
components). Separation of this mixture on plate (silica gel:benzene:EtOAc,
10:4) gave band 1 (Rf 0.45: 38 mg) identified as starting material. Band 2 (Rf
0.32: 42 mg; 43%) which crystallized from ethylacetate as des-Nmethylisoacronycine,
MP: 293°-295°C. Band 3 (Rf 0.10; 29 mg, 29%)
crystallized from ethyl acetate as des-N-methylacronycine. MP: 237°-240°C.
Des-N-methylacronycine (14 mg) was dissolved in dry acetone (10 ml),
anhydrous K2CO3 (1 g), and MeI (2 ml) added and the mixture refluxed for 11
hours. The solution was filtered and the solvents evaporated to give a solid
(12 mg) which after purification on TLC, gave acronycine which crystallized
from aqueous MeOH, MP: 171°-173°C. This product showed identical U.V. and
Rf characterization when compared with acronycine and had an accurate mass measurement of 321.1368. C20H19NO3, required: 321.1364. UV and 1H NMR
spectrum confirmed the structures of all described compounds.
Therapeutic Function
Antineoplastic
General Description
Yellow powder.
Air & Water Reactions
Water insoluble.
Reactivity Profile
Amines, like Acronine, are weak chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides.
Health Hazard
ACUTE/CHRONIC HAZARDS: When heated to decomposition Acronine emits toxic fumes.
Fire Hazard
Flash point data for Acronine are not available. Acronine is probably combustible.
Acronine Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Shaanxi Pioneer Biotech Co., Ltd . | +8613259417953 | sales@pioneerbiotech.com | China | 3000 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 9116 | 58 |
| Hu Bei Jiutian Bio-medical Technology CO.,Ltd | 027-88013699 17354350817 | Ryan@jiutian-bio.com | China | 7433 | 58 |
| Sichuan Wei Keqi Biological Technology Co., Ltd. | 028-81700200 18116577057 | 3003855609@qq.com | China | 7892 | 56 |
| Shanghai Yongye Biotechnology Co., Ltd. | 86-021-61559134 15921386130 | 3423497944@qq.com | China | 8147 | 55 |
| EMMX Biotechnology LLC | 888-539-0666 | info@emmx.com | United States | 8449 | 60 |
| Shanghai ChengShao Biological Technology Co., Ltd. | 021-61847300 13341622919 | shcss01@163.com | China | 4809 | 58 |
| Zhejiang Lianshuo Biotechnology Co., Ltd. | 18616526224 | lianshuo@vip.126.com | China | 9610 | 58 |
7008-42-6(Acronine)Related Search:
1of4