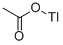THALLIUM(I) ACETATE
- CAS No.
- 563-68-8
- Chemical Name:
- THALLIUM(I) ACETATE
- Synonyms
- TlOAc;THALLOUS ACETATE;THALLIUM ACETATE;thalliumethanoate;Thallium(Ⅰ)acetate;thalliummonoacetate;rcrawastenumberu214;THALLIUM(I) ACETATE;THALLIUM(+1)ACETATE;Thallium monoacetate
- CBNumber:
- CB7350283
- Molecular Formula:
- C2H3O2Tl
- Molecular Weight:
- 263.43
- MDL Number:
- MFCD00013045
- MOL File:
- 563-68-8.mol
| Melting point | 124-128 °C |
|---|---|
| Density | 3.77 |
| storage temp. | Store at RT. |
| solubility | soluble in H2O, ethanol |
| form | Powder/Solid |
| color | White to off-white |
| Water Solubility | Soluble in water, alcoholSoluble in cold water, ethanol, chloroform and ether. Sparingly soluble in hot water. Insoluble in acetone. |
| Sensitive | Hygroscopic |
| Merck | 14,9257 |
| BRN | 3693914 |
| CAS DataBase Reference | 563-68-8(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | Q2901889VM |
| EPA Substance Registry System | Thallium(I) acetate (563-68-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS06,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H300+H330-H373-H411 | |||||||||
| Precautionary statements | P260-P264-P270-P273-P304+P340+P310-P314 | |||||||||
| Hazard Codes | T+,N | |||||||||
| Risk Statements | 26/28-33-51/53 | |||||||||
| Safety Statements | 13-28-45-61 | |||||||||
| RIDADR | UN 1707 6.1/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | AJ5425000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29152900 | |||||||||
| NFPA 704 |
|
THALLIUM(I) ACETATE Chemical Properties,Uses,Production
Chemical Properties
White to off-white fine crystalline powder
Uses
Acetic Acid Thallium(1+) Salt, is a Thalum salt, considere to be a hazardous material.It can be used as in Chemical Synthesis, Micro/NanoElectronics, and Materials Science.
Uses
Thallium acetate is used in high specific gravity solutions to separate ore constituents by flotation. It was formerly used as a depilating agent by dermatologists via oral administration in cases of ringwormof the scalp, causinga number ofdeathsand poisonings.Thallium(I) acetate (0.1M) is used to enhance phosphorescence emission on laserinduced room-temperature phosphorimetry.
Uses
Rodenticide, insecticide; fireworks dye; in optical glass; depilatory.
Definition
ChEBI: An acetate salt comprising equal numbers of acetate and thallium ions.
Production Methods
Thallium acetate is prepared from thallium(I) hydroxide or carbonate and acetic acid, which are then evaporated and recrystallized from alcohol.
General Description
White odorless crystalline solid. Density 3.68 g / cm3. A dangerous poison.
Air & Water Reactions
Deliquescent. Soluble in water.
Reactivity Profile
THALLIUM(I) ACETATE gives aqueous solutions that are basic (neutralize acids). These neutralizations generate only a little heat. Neither a strong reducing agent nor oxidizing agent, but can serve as both.
Health Hazard
Thallium is one of the more toxic elements both as an acute and a chronic poison. Effects of exposure are cumulative and onset of symptoms may be delayed 12 to 24 hours. May be fatal if inhaled, ingested or absorbed through the skin. Irritating to skin and eyes. Readily absorbed through the skin and digestive tract. Ingestion of soluble thallium compounds has caused many deaths. Ingestion of sublethal quantities may cause nausea, vomiting, diarhea, abdominal pain, and bleeding from the gut accompanied or followed by drooping eyelids, crossed eyes, weakness, numbness, tingling of arms and legs, trembling, tightness and pain in the chest. Loss of hair may occur in two to three weeks. Severe intoxication may cause prostration, rapid hearbeat, convulsions, and psychosis. Some effects may be permanent.
Safety Profile
Human poison by ingestion. Experimental poison by ingestion, intravenous, intraperitoneal, and subcutaneous routes. An experimental teratogen. Mutation data reported. When heated to decomposition it emits toxic fumes of T1. See also THALLIUM COMPOUNDS.