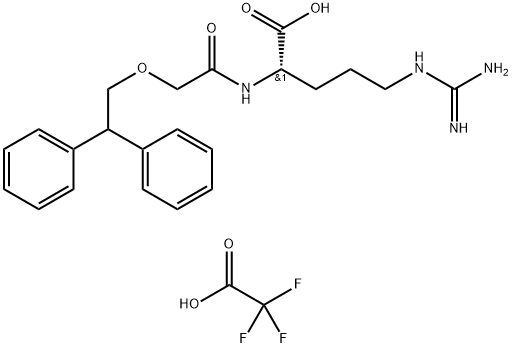
SB 290157 trifluoroacetate salt
- CAS No.
- 1140525-25-2
- Chemical Name:
- SB 290157 trifluoroacetate salt
- Synonyms
- SB290157 (trifluoroacetate);SB 290157 trifluoroacetate salt;SB 290157 - CAS 1140525-25-2 - Calbiochem;SB 290157 TRIFLUOROACETATE; SB-290157 TRIFLUOROACETATE;N2-(2-(2,2-diphenylethoxy)acetyl)-L-arginine, 2,2,2-trifluor;N2-(2-(2,2-diphenylethoxy)acetyl)-L-arginine (trifluoroacetate);(2S)-5-(diaminomethylideneamino)-2-[[2-(2,2-diphenylethoxy)acetyl]amino]pentanoic acid;SB290157,SB-290157 trifluoroacetate,Inhibitor,inhibit,SB 290157,Complement System,SB-290157,SB290157 trifluoroacetate
- CBNumber:
- CB82537362
- Molecular Formula:
- C24H29F3N4O6
- Molecular Weight:
- 526.51
- MDL Number:
- MFCD04974198
- MOL File:
- 1140525-25-2.mol
- MSDS File:
- SDS
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
SB 290157 trifluoroacetate salt price More Price(21)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SML1192 | SB290157 trifluoroacetate salt ≥97% (HPLC) | 1140525-25-2 | 5mg | $103 | 2024-03-01 | Buy |
| Sigma-Aldrich | 559410 | SB 290157 - CAS 1140525-25-2 - Calbiochem | 1140525-25-2 | 10mg | $166 | 2024-03-01 | Buy |
| Cayman Chemical | 15783 | SB 290157 (trifluoroacetate salt) ≥95% | 1140525-25-2 | 1mg | $44 | 2024-03-01 | Buy |
| Cayman Chemical | 15783 | SB 290157 (trifluoroacetate salt) ≥95% | 1140525-25-2 | 5mg | $87 | 2024-03-01 | Buy |
| Sigma-Aldrich | SML1192 | SB290157 trifluoroacetate salt ≥97% (HPLC) | 1140525-25-2 | 25mg | $403 | 2024-03-01 | Buy |
SB 290157 trifluoroacetate salt Chemical Properties,Uses,Production
Uses
SB 290157 Trifluoroacetate Salt is a selective, high affinity, competitive antagonist of anaphylatoxin C3a receptor.
General Description
A non-peptide with anti-inflammatory properties that acts as a selective, high affinity, competitive antagonist of the anaphylatoxin C3a receptor (C3aR; IC50 = 200 nM). Does not antagonize C5aR or other chemotactic G-protein coupled receptors. Blocks C3a-induced internalization of C3aR in human neutrophils and C3a-induced Ca2+ mobilization in basophilic leukemia RBL-2H3 cells expressing murine, guinea pig, or human C3aR (IC50 = 7, 12.5, and 27.7 nM, respectively). Also blocks C3a-mediated ATP release from guinea pig platelets (IC50 = 30 nM).
Biological Activity
sb 290157 is a c3ar antagonist.the anaphylatoxin c3a, a potent chemotactic peptide and inflammatory mediator, binds to and activates a g-protein-coupled receptor. molecular cloning of the c3ar has facilitated the identification of the non-peptide c3ar antagonists.
Biochem/physiol Actions
Target IC50: 200 nM against the anaphylatoxin C3a receptor; 7, 12.5, and 27.7 nM, in blocking C3a-induced internalization of C3aR in human neutrophils and C3a-induced Ca2+ mobilization in basophilic leukemia RBL-2H3 cells expressing murine, guinea pig, or human C3aR
in vitro
sb 290157 was a competitive antagonist of 125i-c3a and binding to rat basophilic leukemia (rbl)-2h3 cells expressing the human c3ar , with an ic50 of 200 nm. sb 290157 could also block the c3a-induced c3ar internalization concentration-dependently and c3a-induced ca21 mobilization in rbl-c3ar cells and human neutrophils. sb 290157 was founf to be selective for the c3ar in that it did not antagonize the c5ar or six other chemotactic g protein-coupled receptors. in addition, sb 290157 could also inhibit c3a induced ca21 mobilization of rbl-2h3 cells expressing the mouse and guinea pig c3ars [1].
in vivo
in animal models, sb 290157 was able to inhibit neutrophil recruitment in a guinea pig lps-induced airway neutrophilia model and decrease paw edema in a rat adjuvant-induced arthritis model [1].
IC 50
200 nm
storage
Desiccate at RT
References
[1] r. s. ames, d. lee, j. j. foley, et al. identification of a selective nonpeptide antagonist of the anaphylatoxin c3a receptor that demonstrates antiinflammatory activity in animal models. journal of immunology 166(10), 6341-6348 (2001).
SB 290157 trifluoroacetate salt Preparation Products And Raw materials
Raw materials
Preparation Products
SB 290157 trifluoroacetate salt Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| GL Biochem (Shanghai) Ltd. | +86-021-61263370 +86-18901917034 | CHINA | 3904 | 58 | |
| InvivoChem | +1-708-310-1919 +1-13798911105 | sales@invivochem.cn | United States | 6393 | 58 |
| Wuhan Topule Biopharmaceutical Co., Ltd | +8618327326525 | masar@topule.com | China | 8474 | 58 |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +undefined18621343501 | product@acmec-e.com | China | 33350 | 58 |
| Aladdin Scientific | +1-833-552-7181 | sales@aladdinsci.com | United States | 57511 | 58 |
| Chembest Research Laboratories Limited | 021-20908456 | sales@BioChemBest.com | China | 6010 | 61 |
| VDM Biochemicals | 0330-2528181 | sales@vdmbio.com | United States | 510 | 64 |
| BOC Sciences | 1-631-485-4226; 16314854226 | info@bocsci.com | United States | 14059 | 65 |
| Wuxi Zhongkun Biochemical Technology Co., Ltd. | 0510-85629785 18013409632 | sales@reading-chemicals.com | China | 15185 | 58 |