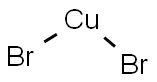Cupric bromide
- CAS No.
- 7789-45-9
- Chemical Name:
- Cupric bromide
- Synonyms
- COPPER BROMIDE;COPPER(II) BROMIDE;Dibromocopper;brominating agent;copperbromide(cubr2);CUPRIC BROMIDE;copperdibromide;Copper(Ⅱ) bromide;COPPER(+2)BROMIDE;Brominated ketone
- CBNumber:
- CB9307690
- Molecular Formula:
- Br2Cu
- Molecular Weight:
- 223.35
- MDL Number:
- MFCD00010970
- MOL File:
- 7789-45-9.mol
- MSDS File:
- SDS
| Melting point | 498 °C(lit.) |
|---|---|
| Boiling point | 900 °C |
| Density | 4.77 g/mL at 25 °C(lit.) |
| Flash point | 900°C |
| storage temp. | Store below +30°C. |
| solubility | 1200g/l |
| form | Crystalline Powder |
| Specific Gravity | 4.77 |
| color | Gray-blue |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| Merck | 14,2629 |
| Exposure limits |
ACGIH: TWA 1 mg/m3 NIOSH: IDLH 100 mg/m3; TWA 1 mg/m3 |
| Stability | Stable. Incompatible with alkali metals, moisture. Reacts violently with potassium. |
| CAS DataBase Reference | 7789-45-9(CAS DataBase Reference) |
| FDA UNII | 1KC430K0ZN |
| NIST Chemistry Reference | copper(II) dibromide(7789-45-9) |
| EPA Substance Registry System | Copper bromide (CuBr2) (7789-45-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS05,GHS07,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H314-H410 | |||||||||
| Precautionary statements | P260-P273-P280-P301+P312-P303+P361+P353-P305+P351+P338 | |||||||||
| Hazard Codes | C,Xi | |||||||||
| Risk Statements | 22-34-36/37/38 | |||||||||
| Safety Statements | 26-36/37/39-45-24/25 | |||||||||
| RIDADR | UN 3260 8/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2827 59 00 | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | III | |||||||||
| Toxicity | LD50 orally in Rabbit: 536 mg/kg | |||||||||
| NFPA 704 |
|
Cupric bromide price More Price(60)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.14658 | Copper(II) bromide for synthesis | 7789-45-9 | 50g | $38.7 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.14658 | Copper(II) bromide for synthesis | 7789-45-9 | 250g | $99.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | 221775 | Copper(II) bromide 99% | 7789-45-9 | 5g | $103 | 2024-03-01 | Buy |
| Sigma-Aldrich | 221775 | Copper(II) bromide 99% | 7789-45-9 | 100g | $138 | 2024-03-01 | Buy |
| Alfa Aesar | 014258 | Copper(II) bromide, Reagent Grade, Cu 28.1% min | 7789-45-9 | 100g | $52.65 | 2024-03-01 | Buy |
Cupric bromide Chemical Properties,Uses,Production
Description
Cupric bromide is a kind of inorganic compound obtained through the reaction between copper oxide and hydrobromic acid. It can be used in laser, generating pulse yellow and green light. The cupric bromide laser is an important technology used in dermatology for the treatment of pigmented lesion and vascular lesions. It can also be used in living radical polymerization and as an intensifier in photographic processing. It is also a brominating agent used in organic synthesis. In addition, it is a kind of highly efficient catalyst in the direct alkynylation of azoles.
Chemical Properties
Copper(II) bromide, CuBr2, [7789-45-9], MW 223.36, MP 498°C, d 4.77, is a black, deliquescent, monoclinic, crystalline material that obtains from warm aqueous solution. At temperatures below 29°C, the green tetrahydrate is produced. Copper(II) bromide is very soluble in water and soluble in alcohol and acetone.
Uses
Copper(II) bromide is used as a catalyst in organic reactions, as an intensifier in photography, and as a brominating agent.
Preparation
Copper(II) bromide is most easily prepared by neutralization of copper(II) oxide, carbonate, or hydroxide with hydrobromic acid. It can also be produced by oxidation of copper metal with bromine water or by reaction of bromine solutions in alcohol with copper powder.
References
Shen, Youqing, Shiping Zhu, and Robert Pelton. Macromolecules 34.10 (2001): 3182-3185.
Rothfleisch, Jeremy E., et al. Dermatologic clinics 20.1 (2002): 1- 18.
Huang, Jianhui, Simon JF Macdonald, and Joseph PA Harrity. Chemical Communications 4 (2009): 436-438.
Besselièvre, François, and Sandrine Piguel. Angewandte Chemie International Edition 48.50 (2009): 9553-9556.
Chemical Properties
Black crystal
Uses
As intensifier in photography; as brominating agent in organic synthesis; as humidity indicator; as wood preservative; in solid-electrolyte battery; as stabilizer for acetylated polyformaldehyde.
Uses
Copper(II) bromide is used in photographic processing as an intensifier and as a brominating agent in organic synthesis. It is also used in the coupling of o-iodophenols and aryl acetylenes avoiding the use of palladium. It finds application in the preparation of NK1/NK2 receptor antagonists used in the regulation of tachykinin.
Uses
Some reported applications of copper bromide are:
- catalyst in cross coupling reactions.
- co-catalyst in Sonogashira coupling.
- Lewis acid in enantioselective addition of alkynes.
- reducing agent, when complexed by three molecules of pyridine initiators for the controlled polymerization of styrene, methyl acrylate and methyl methacrylate.
Reductive homocoupling of α-bromo- α- chlorocarboxylates to dimethyl α, α′ dichlorosuccinate derivatives in presence of CuBr/LiOCH3 in methanol has been reported.
General Description
Odorless black solid. Sinks and mixes with water.
Air & Water Reactions
Deliquescent. Water soluble.
Reactivity Profile
Acidic inorganic salts, such as Cupric bromide, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.
Health Hazard
Inhalation of dust causes irritation of throat and lungs. Ingestion of large amounts causes violent vomiting and purging, intense pain, collapse, coma, convulsions, and paralysis. Contact with solutions causes eye irritation; contact with solid causes severe eye surface injury and skin irritation.
Fire Hazard
Special Hazards of Combustion Products: Irritating hydrogen bromide gas may form in fire.
Flammability and Explosibility
Not classified
Purification Methods
Crystallise it twice by dissolving it in water (140mL/g), filtering to remove any Cu2Br2, and concentrating under vacuum at 30o until crystals appear. The cupric bromide is then allowed to crystallise by leaving the solution in a vacuum desiccator containing P2O5 [Hope et al. J Chem Soc 5226 1960, Glemser & Sauer in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol II p 1009 1965].
Cupric bromide Preparation Products And Raw materials
Raw materials
Preparation Products
1of4
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shanghai Bojing Chemical Co.,Ltd. | +86-86-02137122233 +8613795318958 | bj1@bj-chem.com | China | 298 | 55 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Nanjing Finetech Chemical Co., Ltd. | 025-85710122 17714198479 | sales@fine-chemtech.com | CHINA | 885 | 55 |
| Zjartschem | +86-571-8723 8903 jocelynpan@zjarts.com | jocelynpan@zjarts.com | CHINA | 993 | 58 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8823 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Changzhou Carman Chemical Co., Ltd. | 15061933924 | CHINA | 514 | 58 |
View Lastest Price from Cupric bromide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-11-02 | Copper(II) bromide
7789-45-9
|
US $55.00-30.00 / kg | 1 kg/barrel | 98% | 1kg;5kg;10kg;100kg;1000kg... | Zjartschem | |
 |
2023-09-11 | Cupric bromide
7789-45-9
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2022-12-01 | Copper(II) bromide, anhydrous
7789-45-9
|
US $30.00-15.00 / kg | 1kg | 99% | 20MT/month | Shanghai Bojing Chemical Co.,Ltd. |
-

- Copper(II) bromide
7789-45-9
- US $55.00-30.00 / kg
- 98%
- Zjartschem
-

- Cupric bromide
7789-45-9
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Copper(II) bromide, anhydrous
7789-45-9
- US $30.00-15.00 / kg
- 99%
- Shanghai Bojing Chemical Co.,Ltd.
7789-45-9(Cupric bromide)Related Search:
1of4