メトトレキサート水和物 化学特性,用途語,生産方法
外観
黄色~黄褐色, 結晶性粉末~粉末
溶解性
本品0.1g+0.05M炭酸ナトリウム溶液10mlにて澄明水酸化ナトリウム溶液又は、炭酸ナトリウム溶液に溶け、ピリジンに溶けにくく、水、アセトニトリル、エタノール(95)又はジエチルエーテルにはほとんど溶けない。
解説
C20H22N8O5(454.46).2,4,5,6-テトラアミノピリミジン,p-メチルアミノベンゾイルグルタミン酸,2,3-ジブロモプロピオンアルデヒドから合成される.
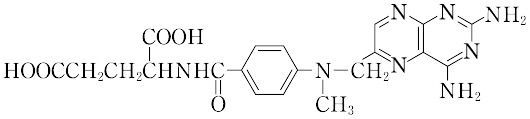
"分解点185~204 ℃.λmax 244,307 nm(0.1 mol L-1 塩酸).水,有機溶媒に不溶,希アルカリに可溶.葉酸類似の構造をもつ代謝きっ抗薬で,それによって核酸とプリンとの生合成を妨げ,抗腫瘍効果を示す.免疫抑制剤として使用される.LD50 146 mg/kg(マウス,経口).[CAS 59-05-2]
森北出版「化学辞典(第2版)
用途
制癌作用及び白血病の研究。
用途
メトトレキサート(Methotrexate)は、葉酸代謝拮抗機序をもち免疫抑制剤に分類される薬剤である。抗悪性腫瘍薬(抗がん剤)、抗リウマチ薬、妊娠中絶薬などとして使用される。
葉酸を活性型葉酸にする酵素の働きを阻止することにより、核酸合成を阻止し、細胞増殖を抑制する。
免疫グロブリン産出、抗体産出、リンパ球増殖などの抑制により、免疫を抑制すると考えられている。また、滑膜組織や軟骨組織の破壊に関係するコラゲナーゼの産出を抑制する。
用途
葉酸代謝阻害作用を示します。
生理機能
メトトレキサートは,葉酸代謝拮抗物質。葉酸誘導体であり,葉酸から四水素葉酸への変化を阻害することにより核酸代謝を阻害し,細胞の核分裂,増殖を抑制する。悪性腫瘍の治療薬として白血病,特に小児白血病や脈絡膜上皮腫に有効であるが,耐性を生じることがある。血液脳関門は通過しにくい。そのほか乾癬症にも用いられる。骨髄に対する作用が強いので,副作用として赤血球増殖抑制,白血球およびリンパ球減少があり,生殖細胞抑制も強く,また免疫反応抑制,出血性腸炎がある。
効能
抗悪性腫瘍薬, 代謝拮抗薬
商品名
メソトレキセート (ファイザー); メソトレキセート (ファイザー); メソトレキセート (ファイザー); メソトレキセート (ファイザー); リウマトレックス (ファイザー)
化学的特性
Methotrexate is an orange-brown crystalline
powder.
使用
Methotrexate is used to treat severe lymphatic leukemia, choriocarcinoma, non-Hodgkin’s
lymphoma, bone carcinoma, as well as head, neck, breast, and lung tumors.
適応症
Methotrexate is approved for use in severe disabling
psoriasis recalcitrant to other less toxic treatments. The
standard regimen is similar to low-dose therapy used
for the treatment of rheumatoid arthritis . Although toxicities are similar to those described in
the treatment of other diseases, hepatic cirrhosis and
unexpected pancytopenia are of special concern given
the chronicity of treatment.
生物学の機能
Although the mechanism of
action of methotrexate in rheumatoid arthritis is unknown, recent studies have shown that methotrexate reversibly
inhibits dihydrofolate reductase, blocking the proliferation of B cells by interfering with DNA synthesis, repair, and
replication. Oral absorption is dose-dependent, being well-absorbed at doses of 7.5–25 mg once a week. At this
dose, oral bioavailability is approximately 60%, and food can delay absorption and reduce peak concentration. The
volume of distribution is 0.4 to 0.8 L/kg. Protein binding is approximately 50%. It is metabolized to active metabolites,
methotrexate polyglutamates and 7-hydroxymethotrexate. Some metabolism occurs by intestinal flora after oral administration.
Methotrexate is actively transported into the urine (80–90% unchanged in the urine within 24 hours) via the folate
transporter, an organic anion transporter. Its elimination half-life is 3 to 10 hours.
獲得抵抗性
Mammalian cells have several mechanisms of resistance
to methotrexate. These include an increase in intracellular
dihydrofolate reductase levels, appearance
of altered forms of dihydrofolate reductase with decreased
affinity for methotrexate, and a decrease in
methotrexate transport into cells. The
relative importance of each of these mechanisms of resistance
in various human tumors is not known.
Cellular uptake of the drug is by carrier-mediated
active transport. Drug resistance due to decreased
transport can be overcome by greatly increasing extracellular
methotrexate concentration, which provides a
rationale for high-dose methotrexate therapy. Since
bone marrow and gastrointestinal cells do not have impaired
folate methotrexate transport, these normal cells
can be selectively rescued with reduced folate, bypassing
the block of dihydrofolate reductase. Leucovorin
(citrovorum factor, folinic acid, 5-formyltetrahydrofolate)
is the agent commonly used for rescue.
一般的な説明
Methotrexate (MTX, Rheumatrex), an antifolate drug used in cancer treatment, has also been used in the disease management of RA since the 1950s. Because of its quicker therapeutic onset among all DMARDs and its demonstrated efficacy, tolerability, and low cost, MTX has been the firstline therapy for RA patients who are not responsive to NSAIDs alone.
Recent findings have indicated that other DMARDs should only be used for patients who are refractory to MTX. At least four anti-inflammatory mechanisms of action have been suggested for MTX’s ability to slow down RA disease progression. First, MTX, being a folate antagonist, prevents antigen-dependent T-cell proliferation by blocking de novo pyrimidine biosynthesis, via a reversible inhibition of dihydrofolate reductase. It also inhibits folate-mediated production of spermine and spermidine in synovial tissue. These polyamines are believed to be the toxic compounds responsible for causing tissue injury in RA. MTX can also reduce intracellular glutathione concentration, thereby altering the cellular redox state that suppresses the formation of reactive oxygen radicals in synovial tissue. Lastly, MTX, similar to sulfasalazine, infliximab, and IL-4, can also inhibit osteoclastogenesis (i.e., bone erosion) in patients with RA, by modulating the interaction of the receptor activator of nuclear factor B, its ligand, and osteoprotegrin.
空気と水の反応
Methotrexate is sensitive to hydrolysis, oxidation and light. Insoluble in water.
反応プロフィール
Methotrexate decomposes in very acidic or alkaline conditions. Methotrexate is incompatible with strong oxidizing agents and strong acids.
危険性
Very toxic. Questionable carcinogen.
火災危険
Flash point data for Methotrexate are not available; however, Methotrexate is probably combustible.
生物活性
Cytotoxic agent. Inhibits thymidylate synthetase and de novo purine synthesis. Potent folic acid antagonist; inhibits dihydrofolate reductase. Also inhibits Ras carboxyl methylation in DKOB8 cells, leading to decreased p44 and Akt activation.
作用機序
Methotrexate is a folic acid antagonist structurally designed to compete successfully with 7,8-DHF for the DHFR enzyme. The direct inhibition of DHFR causes cellular levels of 7,8-DHF to build up, which in turn results in feedback (indirect) inhibition of thymidylate synthase. Methotrexate also is effective in inhibiting glycine amide ribonucleotide (GAR) transformylase , a key enzyme in the synthesis of purine nucleotides. Take note of the structural differences between methotrexate and DHF, because these differences will be important to an understanding of the chemical mechanism of this anticancer agent.
薬理学
Methotrexate is a folate antimetabolite that inhibits dihydrofolate
reductase and other folate-dependent enzymes
in cells. At the low doses used in the therapy
of rheumatoid arthritis,methotrexate appears to be acting
more as an antiinflammatory agent than as an immunosuppressant.
Methotrexate inhibits folate-dependent
enzymes involved in adenosine degradation,
increasing concentrations of extracellular adenosine.
Adenosine acts via cell surface receptors to inhibit the
production of inflammatory cytokines such as TNF-α
and IFN-γ.Methotrexate also decreases the production
of inflammatory prostaglandins and proteases, though a
direct action on the COX enzymes has not been noted.
臨床応用
Methotrexate is part of curative combination
chemotherapy for acute lymphoblastic leukemias,
Burkitt’s lymphoma, and trophoblastic choriocarcinoma.
It is also useful in adjuvant therapy of breast carcinoma;
in the palliation of metastatic breast, head, neck,
cervical, and lung carcinomas; and in mycosis fungoides.
High-dose methotrexate administration with leucovorin
rescue has produced remissions in 30% of patients
with metastatic osteogenic sarcoma.
Methotrexate is one of the few anticancer drugs that
can be safely administered intrathecally for the treatment
of meningeal metastases. Its routine use as prophylactic
intrathecal chemotherapy in acute lymphoblastic
leukemia has greatly reduced the incidence
of recurrences in the CNS and has contributed to the
cure rate in this disease. Daily oral doses of methotrexate
are used for severe cases of the nonneoplastic skin
disease psoriasis, and methotrexate
has been used as an immunosuppressive agent in severe
rheumatoid arthritis.
副作用
In the low-dose regimen used for rheumatoid arthritis,
most side effects of methotrexate are mild and can be
managed by temporarily stopping the drug or reducing
the dose. These include nausea, stomatitis, GI discomfort,
rash, diarrhea, and headaches. Changes in liver
aminotransferases and mild to moderate immunosuppression
have been reported in rheumatoid arthritis patients
taking methotrexate. Severe toxicity is possible
but rare and may be a function of drug accumulation.
These effects include hepatotoxicity progressing to cirrhosis,
pneumonitis progressing to pulmonary fibrosis,
and bone marrow depression with anemia, leukopenia,
and thrombocytopenia. Folic acid supplementation is often
used to alleviate certain side effects of methotrexate
therapy (stomatitis, GI irritation, hematopoietic effects)
but may also contribute to resistance to this therapy.
職業ばく露
Methotrexate is an alkaloid anticancer
drug available in tablet or injectable liquid form. A chemotherapy drug that interferes with DNA and RNA synthesis.
It is also an insect chemosterilant.
代謝
Methotrexate can be given orally in the treatment of breast, head and neck, and various lung cancers as well as in non-Hodgkin's lymphoma. The sodium salt form also is marketed for IV, intramuscular, intra-arterial, or intrathecal injection. Oral absorption is dose-dependent and peaks at 80 mg/m2 because of site saturation. The monoglutamate tail of methotrexate permits active transport into cells, with carrier-mediated transport predominating at serum concentration levels lower than 100 μM. Once inside the cell, methotrexate undergoes a polyglutamation reaction that adds several anionic carboxylate groups to trap the drug at the site of action. Polyglutamation is more efficient in tumor cells than in healthy cells and, therefore, may promote selective toxicity of this drug. Cancer cells can become resistant to methotrexate over time which may involve impaired transport across tumor cell membranes, enhanced efflux from the tumor cell, and attenuated polyglutamation rates. The polyglutamated drug will be hydrolyzed back to the parent structure before renal elimination. Up to 90% of an administered dose of methotrexate is excreted unchanged in the urine within 24 hours.
輸送方法
UN1544 Alkaloids, solid, n.o.s. or Alkaloid salts,
solid, n.o.s. poisonous, Hazard Class: 6.1; Labels: 6.1-
Poisonous materials, Technical Name Required. UN2811
Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-
Poisonous materials, Technical Name Required.
純化方法
Most common impurities are 10-methylpteroylglutamic acid, aminopterin and pteroylglutamic acid. Purify it by chromatography on Dowex-1 acetate, followed by filtration through a mixture of cellulose and charcoal. It has been recrystallised from aqueous HCl or by dissolution in the minimum volume of N NaOH and acidified until precipitation is complete, filter or better collect by centrifugation, wash with H2O (also by centrifugation) and dry at 100o/3mm. It has UV: max at 244 and 307nm ( 17300 and 19700) in H2O at pH 1; 257, 302 and 370nm ( 23000, 22000 and 7100) in 2O at pH 13. [Momle Biochemical Preparations 8 20 1961, Seeger et al. J Am Chem Soc 71 1753 1949.] It is a potent inhibitor of dihydrofolate reductase and is used in cancer chemotherapy. [Blakley The Biochemistry of Folic Acid and Related Pteridines, North-Holland Publ Co., Amsterdam, NY, pp157-163 1969, Beilstein 26 IV 3833.] It is CARCINOGENIC; HANDLE WITH EXTREME CARE.
不和合性
Combustible. Compounds of the carboxyl group react with all bases, both inorganic and
organic (i.e., amines) releasing substantial heat, water and a
salt that may be harmful. Incompatible with arsenic compounds (releases hydrogen cyanide gas), diazo compounds,
dithiocarbamates, isocyanates, mercaptans, nitrides, and
sulfides (releasing heat, toxic, and possibly flammable
gases), thiosulfates and dithionites (releasing hydrogen sulfate and oxides of sulfur). Incompatible with oxidizers
(chlorates, nitrates, peroxides, permanganates, perchlorates,
chlorine, bromine, fluorine, etc.); contact may cause fires or
explosions. Keep away from alkaline materials, strong bases,
strong acids, oxoacids, epoxides, light, UV, moisture.
廃棄物の処理
It is inappropriate and possibly dangerous to the environment to dispose of expired or
waste drugs and pharmaceuticals by flushing them down
the toilet or discarding them to the trash. Household quantities of expired or waste pharmaceuticals may be mixed
with wet cat litter or coffee grounds, double-bagged in
plastic, discard in trash. Larger quantities shall carefully
take into consideration applicable DEA, EPA, and FDA
regulations. If possible return the pharmaceutical to the
manufacturer for proper disposal being careful to properly
label and securely package the material. Alternatively, the
waste pharmaceutical shall be labeled, securely packaged and transported by a state licensed medical waste contractor
to dispose by burial in a licensed hazardous or toxic waste
landfill or incinerator.
予防処置
Methotrexate is teratogenic and is contraindicated duringpregnancy and breast-feeding. Prior to attemptingpregnancy, women should wait at least one menstrualcycle and men at least 3 months after discontinuing thisdrug. Additional contraindications to methotrexate administrationinclude kidney, liver, and lung disease;moderate to high alcohol use; immunodeficiency; blooddyscrasias; and hypersensitivity. Elderly persons may be at increased risk for toxicity because of decreased renaland hepatic function.
Methotrexate clearance can be decreased by thecoadministration of NSAIDs; however, this not usuallya problem with the low doses of methotrexate used totreat arthritis. Methotrexate can be displaced fromplasma protein binding sites by phenylbutazone, phenytoin,sulfonylureas, and sulfonamides and certain otherantibiotics. The antifolate effects of methotrexate areadditive with those of other folate-inhibitory drugs,such as trimethoprim.
メトトレキサート水和物 上流と下流の製品情報
原材料
準備製品
(1aR)-1,1aα,1bβ,4,4a,7aα,7b,8,9,9a-デカヒドロ-4aβ,7bα,9β,9aα-テトラヒドロキシ-3-(ヒドロキシメチル)-1,1,6,8α-テトラメチル-5H-シクロプロパ[3,4]ベンゾ[1,2-e]アズレン-5-オン