ヒドラジン (無水) 化学特性,用途語,生産方法
解説
ヒドラジン,H2N-NH2(市販品の多くは一水和物)と,そのHを炭化水素基で置換した化合物(ヒドラジン類)をいう.【Ⅰ】N2H4(32.05)(一水和物は,N2H4・H2O(50.06)):体系名はジアザン(diazane).硫酸ヒドラジニウムにNaOHを反応させた後,N2 中で蒸留すると一水和物が得られる.これを脱水すると無水物も得られる.また,直接Rasching法で,ゼラチンなどを制御剤として,NH3または尿素を,NaClOまたはさらし粉とNa2CO3の混合物とをアルカリ水溶液中で酸化すると得られる.気体では,H2N-NH2分子.N-N約1.45 Å,N-H約1.04 Å.∠N-N-H約112°.両方のH-N-H面の二面角約90~95°.単斜晶系.Hは隣接分子のNと水素結合している.N-N約1.45 Å,N…H約3.19~3.30 Å.無水物は,アンモニア臭のある液体で,空気中で発煙する.融点2.0 ℃,沸点113.5 ℃.密度1.00 g cm-3(25 ℃).180 ℃ で N2 とNH3に分解する.一水和物も発煙性の液体で,沸点118 ℃.密度約1.03 g cm-3(20 ℃).いずれも水,エタノールに易溶,クロロホルムやエーテルに不溶.塩基で,K1b 8.5×10-7,Kb2 8.9×10-15(25 ℃).還元性が大きい.塩,S,Pなどを溶かす.無水ヒドラジンと酸素または空気の混合物は加熱すると燃焼する.猛毒で皮膚をおかす.腐食性も大きく,コルク,ゴム,ガラスなども侵す.還元剤,無機物の溶媒,発泡剤,農薬や医薬品の製造過程での使用,ロケット燃料の原料,清缶剤などに用いられる.
用途
ロケット燃料(無水ヒドラジン),一般的に用いられるヒドラジン水和物(CAS番号:7803-57-8)の用途は発泡剤原料,清缶剤?水処理剤,工業薬品合成原料,農薬合成原料,医薬合成原料 (NITE CHRIP)
製法
いくつかの方法が開発されており、キーとなるポイントは、NーNの単結合を形成する過程にある。
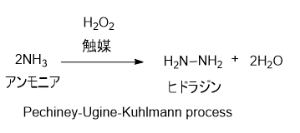
1. パーオキシドプロセス (別名:Pechiney-Ugine-Kuhlmann process)
アンモニアとを触媒存在下で反応させる方法。副生成物として塩類を出さないことが特徴。
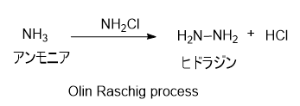
 2. Olin Raschig プロセス
2. Olin Raschig プロセス
とアンモニアを混合することによりを生成し、続いてモノクロラミンとアンモニアが反応することでヒドラジンが生じる。副生成物としてNaClがでる。
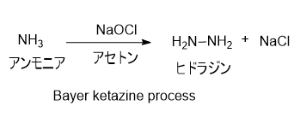
 3. Bayer ketazine process
3. Bayer ketazine process
パーオキシドプロセスが開発される前に工業化されていた方法。次亜塩素酸ナトリウムとアンモニアを中で反応させることで合成する。
説明
Hydrazine sulphate, hydrobromide and hydrochloride
have been reported to be occupational sensitizers,
mainly in soldering flux.
性質
無水ヒドラジンは無色、発煙性、アンモニア臭の油状液体。空気中では発煙する。強熱すると爆発する。水に易溶。弱い塩基でヒドラジニウム塩(一般式N2H5XおよびN2H6X2、Xは1価の陰性原子または基)をつくる。硫酸塩N2H6SO4は水に溶けにくい。水溶液は還元作用があり、銀塩溶液から銀を沈殿させ、鉄(Ⅲ)塩を鉄(Ⅱ)塩に変える。溶存酸素により窒素を生じる。酸化剤となることもある。
化学的特性
HYDRAZINE, colorless, fuming liquid, decomposes when heated above 350 °C at atmospheric pressure into N2 and NH2, also decomposes in presence of a catalyst (e.g., platinum) into N2 and NH3. Hydrazine burns when ignited in air with a violet-colored flame. The compound is soluble in all proportions with H2O and is soluble in alcohol. Hydrazine forms a hydrate with one molecule of H2O. Upon moderate heating or in a vacuum, the hydrate yields hydrazine and H2O. Hydrazine is a base slightly weaker than NH4OH.
物理的性質
Colorless, mobile, fuming liquid; ammoniacal odor; density 1.0045 g/mL at25°C; refractive index 1.46044 at 22°C; solidifies at 2°C to a white crystallinesolid; boils at 113.5°C; flash point 52°C; burns with a violet flame; vapor pres-sure 14.4 torr at 25°C; critical temperature 379.85°C; critical pressure 145atm; surface tension 66.67 dyne/cm at 25°C; dielectric constant 51.7 at 25°C;viscosity 0.876 centipoise at 25°C; very soluble in water; forms an azeotropewith water at molar composition of 58.5% hydrazine: 41.5% water (71.48%:28.52% by weight), the azeotrope with water boils at 120.5°C; forms hydrazinehydrate at 1:1 molar concentration in water; soluble in alcohols and other polar solvents; pKa 8.1 at 25°C.
使用
Hydrazine is used as a high-energy rocket fuel, as a
reducing agent, and for preparing organic hydrazine derivatives.
The propellant grade of commercial hydrazine is more
than 97.7% active. It is also used as an oxygen scavenger in
boiler water. Hydrazine has also been used as an experimental
drug for treating tuberculosis and sickle cell anemia.
調製方法
Hydrazine is a colorless, fuming, oily liquid with an
ammonia-like odor. It should be stored in glass containers
in a cool, dark place.
Hydrazine is prepared commercially by the Raschig and
the urea processes. The Raschig method involves reacting
sodium hypochlorite with excess ammonia, flash boiling to
recover dilute hydrazine, and fractionating to produce the
hydrate. In the urea process, urea is oxidized with hypochlorite
to produce the hydrate. Both anhydrous hydrazine and the
hydrate are fuming, strongly basic (pKb1=5.52), colorless
liquids. Hydrazine may ignite under various circumstances
(e.g., on contact with rust) and it decomposes violently in
contact with oxidizing materials. It is usually stored under
nitrogen to reduce the flammability hazard and to maintain
purity.
定義
A colorless
liquid that can be prepared by the oxidation
of ammonia with sodium
chlorate(I) or by the gas phase reaction of
ammonia with chlorine. Hydrazine is a
weak base, forming salts (e.g. N
2H
4.HCl)
with strong acids. It is a powerful reducing
agent, reducing salts of the noble metals to
the metal. Anhydrous hydrazine ignites
spontaneously in oxygen and reacts violently
with oxidizing agents. The aqueous
solution, hydrazine hydrate, has been used
as a fuel for jet engines and for rockets.
製造
窒素と水素の化合物。アンモニアまたは尿素を次亜塩素酸塩で酸化するラーシッヒ法で製造される。
NH3+NaOCl―→NH2Cl+NaOH
NH2Cl+NH3+NaOH →N2H4+NaCl+H2O 最終的にヒドラジン一水和物の濃水溶液として得られる。重金属イオンが存在すると収量が低下するので、キレート剤、にかわなどを加える。次亜塩素酸塩のかわりに塩素とアセトンを用いるバイヤー法もある。
一般的な説明
Colorless liquid with an ammonia-like odor. A violent poison. Causes delayed eye irritation. Very corrosive, attacks glass, rubber, and cork. Corrodes molybdenum steels such as Allegheny stainless 316.
It is a strong reducing agent and a flammable liquid and vapour. Hydrazine is a useful building block in organic synthesis of pharmaceuticals and pesticides. There are many kinds of hydrazine compounds, including hydrazine, 1,1-dimethylhydrazine, and 1,2-dimethylhydrazine. Small amounts of hydrazine occur naturally in plants. Most hydrazines are manufactured for use as rocket propellants and fuels, boiler water treatments, chemical reactants, medicines, and in cancer research. Hydrazines are highly reactive and easily catch fire.
空気と水の反応
Fumes in air. Water soluble.
反応プロフィール
HYDRAZINE are strongly basic and are powerful reducing agents. Note that a 64% solution corresponds to the composition hydrazine hydrate (N2H4.H2O). Spontaneous ignition can occur with hydrogen peroxide and nitric acid. Contact with metallic oxide surfaces may lead to flaming decomposition [Haz. Chem. Data (1966)]. The reaction between 2,4-dinitrochlorobenzene and hydrazine hydrate shattered the reaction flask [Wischmeyer 1967]. Spontaneous ignition occurs when nitrous oxide and hydrazine are mixed [Mellor 8, Supp. 2:214(1967)]. Potassium and sodium dichromate react explosively with hydrazine [Mellor 11:234(1946-1947)]. Hydrazine hydrate reacts with stannous chloride to give stannous dihydrazinechloride, which decomposes explosively when heated [Mellor 7:430(1946-1947)]. Explodes during distillation if traces of air are present. Affected by UV and metal ion catalysis [Merck, 11th ed., 1989].
危険性
Severe explosion hazard when exposed to
heat or by reaction with oxidizers. Toxic by ingestion, inhalation, and skin absorption; strong irritant
to skin and eyes; a confirmed carcinogen.
健康ハザード
Hydrazine is extremely destructive to the tissues of the mucous membranes and upper
respiratory tract, eyes, and skin. Skin contact with the liquid can result in severe burns;
hydrazine is readily absorbed through the skin, leading to systemic effects, which may
include damage to the liver, kidney, nervous system, and red blood cells. Hydrazine
vapor is irritating to the nose, throat, and respiratory tract, and inhalation of high
concentrations may be fatal as a result of spasm, inflammation, chemical pneumonitis,
and pulmonary edema. Symptoms of exposure may include a burning sensation,
coughing, wheezing, laryngitis, shortness of breath, headache, nausea, and vomiting.
Hydrazine vapor is extremely irritating to the eyes and can cause temporary blindness.
Eye contact with the liquid can result in severe burns and permanent damage. Hydrazine
is not considered to have adequate warning properties.
Hydrazine is listed by IARC in Group 2B "possible human carcinogen" and is classified
as a "select carcinogen" according to the criteria of the OSHA Laboratory Standard.Chronic exposure to subacute levels of hydrazine can cause lethargy, vomiting,
tremors, itching and burning of the eyes and skin, conjunctivitis, and contact
dermatitis. Hydrazine has been found to exhibit reproductive and developmental
toxicity in animal tests.
火災危険
Flammable/combustible material. May be ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
燃焼性と爆発性
Hydrazine is a flammable liquid (NFPA rating = 3) over a very broad range of vapor
concentrations (4.7 to 100%). Hydrazine may undergo autoxidation and ignite
spontaneously when brought in contact with porous substances such as rusty
surfaces, earth, wood, or cloth. Fires should be extinguished with water spray,
carbon dioxide, or dry chemical extinguishers.
使用用途
ヒドラジンは、常温での保温が可能であり、ロケットエンジンの燃料(推進剤)、人工衛星や宇宙探査機の姿勢制御用推進器の燃料として用いられています。
その他様々な化学製品、例えば、軟質・硬質フォーム、、殺菌剤、除草剤、および雑草や害虫駆除用の農薬として農業用途でも使用されています。
また、ヒドラジン水和物は主に、プラスチック発泡剤製造用、脱酸素剤、脱炭酸ガス剤、pH調整、還元剤、重合触媒、ボイラー水処理、廃水からの鉄除去などに使用されております。
人への影響
ヒトへの急性暴露によって中枢神経系、肝臓、腎臓に影響を及ぼすことが知られている。 皮膚及び眼への刺激性、アレルギー性接触性皮膚炎の報告もあるため、使用には注意が必要である。
発がん性
Hydrazine and hydrazine sulfate are reasonably anticipated to be human carcinogens based on sufficient evidence of carcinogenicity from studies in experimental animals.
環境運命予測
Hydrazine can be found in the environment in small quantities
and is a component of tobacco smoke. However, hydrazine is
primarily an industrial chemical that enters the environment by
emissions from its use as an aerospace fuel and from industrial
facilities that manufacture, process, and/or use this chemical.
Treatment and disposal of wastes containing hydrazine also
contribute to environmental concentrations. However, hydrazine
rapidly degrades in the environment and is rarely
encountered outside the industrial setting.
貯蔵
work
with hydrazine should be conducted in a fume hood to prevent exposure by
inhalation, and splash goggles and impermeable gloves should be worn at all times
to prevent eye and skin contact. Hydrazine should be used only in areas free of
ignition sources. Hydrazine should be stored under nitrogen in containers placed in
secondary containers in areas separate from oxidizers and acids.
純化方法
Hydrazine hydrate is dried by refluxing with an equal weight of KOH pellets for 3hours, then distilled from fresh solid NaOH or BaO in a current of dry N2. Use stainless steel or copper equipment. Hydrazine and its hydrates have VERY IRRITATING and TOXIC vapours and should be used in an efficient fume cupboard. Store in a well-stoppered vessel, preferably under N2. It is a reducing agent. [Schenk in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I pp 469-472 1963.]
不和合性
Hydrazine is a highly reactive reducing agent that forms shock-sensitive, explosive
mixtures with many compounds. It explodes on contact with barium oxide, calcium
oxide, chromate salts, and many other substances. On contact with metal catalysts
(platinum black, Raney nickel, etc.), hydrazine decomposes to ammonia, hydrogen,
and nitrogen gases, which may ignite or explode.
廃棄物の処理
In the event of a spill, remove all ignition sources, soak up the hydrazine with a spill
pillow or absorbent material, place in an appropriate container, and dispose of
properly. Evacuation and cleanup using respiratory protection may be necessary in
the event of a large spill or release in a confined area.
Disposal Excess hydrazine and waste material containing this substance should be placed in
an appropriate container, clearly labeled, and handled according to your institution's
waste disposal guidelines. For more information on disposal procedures, see
Chapter 7 of this volume.
ヒドラジン (無水) 上流と下流の製品情報
原材料
準備製品