알긴산나트륨
|
|
알긴산나트륨 속성
- 녹는점
- 99 °C
- 밀도
- 1.0 g/cm3(Temp: 25 °C)
- 저장 조건
- 2-8°C
- 용해도
- 점성 콜로이드 용액을 형성하는 물에 천천히 용해되며, 에탄올에는 거의 용해되지 않습니다(96%).
- 물리적 상태
- 가루
- 색상
- 흰색에서 황백색까지
- 수소이온지수(pH)
- 6.0-8.0 (10mg/mL in H2O)
- 냄새
- 냄새 없는
- 수용성
- 물에 용해됩니다. 알코올, 클로로포름 및 에테르에 용해되지 않습니다.
- 감도
- Hygroscopic
- Merck
- 14,241
- 안정성
- 안정적인. 강산, 강염기, 강산화제와 호환되지 않습니다.
- LogP
- -2.88
- CAS 데이터베이스
- 9005-38-3(CAS DataBase Reference)
- 위험 및 안전 성명
| 위험품 표기 | Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 36/37/38 | ||
| 안전지침서 | 24/25-36-26 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | AZ5820000 | ||
| F 고인화성물질 | 3 | ||
| TSCA | Yes | ||
| HS 번호 | 39131000 | ||
| 유해 물질 데이터 | 9005-38-3(Hazardous Substances Data) | ||
| 독성 | LD50 oral in rat: > 5gm/kg | ||
| 기존화학 물질 | KE-00492 |
알긴산나트륨 C화학적 특성, 용도, 생산
순도시험
(1) 액성 : 이 품목 0.5g을 취한 다음 물 50mL에 가끔씩 흔들어 섞으면서 소량씩 가한 다음 60~70℃에서 가끔씩 흔들어 섞으면서 20분간 가온하여 균등한 액으로 하고 식힌 다음 액의 pH는 6.0~8.0이어야 한다.
(2) 황산염 : 이 품목 0.1g에 물 20mL를 가하여 풀모양으로 하고 염산 1mL를 가하여 잘 흔들어 섞어 수욕 중에서 수분간 가열하고 식힌 다음 여과한다. 이어서 물 10mL씩으로 비이커를 3회 씻어주고 세액을 앞의 여과지를 사용하여 여과시킨 다음 여액을 모두 합하고 다시 물을 가하여 50mL로 한다. 이 액 10mL를 취한 다음 물을 가하여 50mL로 한 것을 시험용액으로 하여 황산염시험을 한다. 다만, 비교액은 0.01N 황산 0.4mL에 염산(1→4) 1mL 및 물을 가하여 50mL로 한다.
(3) 비소 : 이 품목을 비소시험법에 따라 시험할 때, 그 양은 4.0ppm 이하이어야 한다.
(4) 납 : 이 품목 5.0g을 취하여 원자흡광광도법 또는 유도결합플라즈마발광광도법에 따라 시험할 때, 그 양은 5.0ppm 이하이어야 한다.
(5) 카드뮴 : 이 품목 5.0g을 취하여 원자흡광광도법 또는 유도결합플라즈마발광광도법에 따라 시험할 때, 그 양은 1.0ppm 이하이어야 한다.
(6) 수은 : 이 품목을 수은시험법에 따라 시험할 때, 그 양은 1.0ppm 이하이어야 한다.
(7) 인산염 : 이 품목 0.1g을 물 20mL에 저어 섞으면서 소량씩 가한 후 60~70℃에서 때때로 혼합하면서 20분간 가온해서 균등한 액으로 하고 냉각한 다음 이 액에 질산(1→4) 5mL 및 몰리브덴산암모늄시액 20mL를 가하여 가온할 때, 황색의 침전이 생겨서는 아니 된다.
(8) 세균수 : 이 품목은 「식품의 기준 및 규격」 일반시험법의 미생물시험법 중 세균수(일반세균수)에 따라 시험할 때, 1g당 5,000이하이어야 한다.
(9) 대장균 : 이 품목은 「식품의 기준 및 규격」 일반시험법의 미생물시험법 중 대장균에 따라 시험할 때, 음성(-)이어야 한다.
(10) 살모넬라 : 이 품목은 「식품의 기준 및 규격」 일반시험법의 미생물시험법 중 살모넬라에 따라 시험할 때, 음성(-)이어야 한다.
(11) 진균수 : 이 품목은 「식품의 기준 및 규격」 일반시험법의 미생물시험법 중 진균수에 따라 시험할 때, 제품 1g 당 500 이하이어야 한다.
확인시험
(1) 이 품목 0.5g에 물 50mL를 흔들어 주면서 소량씩 가한 다음 60~70℃에서 가끔씩 흔들어 주면서 20분간 가온시켜서 균등한 액으로 한 후 식힌 액을 시험용액으로 하여 다음의 시험을 한다.
(ⅰ) 시험용액 5mL에 염화칼슘시액 1mL를 가하면 젤리모양의 침전이 생긴다.
(ⅱ) 시험용액 10mL에 황산(1→20) 1mL를 가하면 곧 젤리모양의 침전이 생긴다.
(ⅲ) 시험용액 1mL에 황산암모늄포화용액 1mL를 가할 때, 침전이 생겨서는 아니 된다.
(2) 이 품목을 회화하여 얻은 잔류물은 확인시험법 중 나트륨염의 반응을 나타낸다.
정량법
미리 유리여과기(1G4)를 80℃에서 30분간 감압건조하고 데시케이타안에서 방냉시킨 다음 무게를 정밀히 달아둔다. 이 품목을 건조시킨 다음 약 0.5g을 정밀히 달아 수산화나트륨용액(1→25) 10mL를 가하여 용해시킨 후 물 90mL를 가해주고 필요하면 여과한다. 이 액에 염산(1→3) 15mL 및 90% 에탄올 100mL를 가하여 잘 흔들어 섞어준 다음 2시간 방치하고 원심분리한 후 상등액을 버리고 다시 90% 에탄올 10mL를 가하여 잘 흔들어 섞어준 다음 원심분리한 후 상등액은 버린다. 이 조작을 상등액이 염화물반응을 나타내지 않을 때까지 반복한다. 여기서 얻어진 침전물을 90% 에탄올을 사용하여 유리여과기로 여과한다. 잔류물을 아세톤으로 씻어준 후 80℃에서 1시간 감압건조하고 데시케이타 안에서 방치한 다음 무게를 정밀히 달고 다음 계산식에 따라 함량을 구한다.
강열잔류물
이 품목을 105℃에서 4시간 건조한 다음 약 1g을 정밀히 달아 강열잔류물시험법에 따라 시험할 때, 그 잔류물은 33~37%이어야 한다.
개요
Sodium alginate is the sodium form of alginate. Alginate is a linear, anionic polysaccharide consisting of two form of 1, 4-linked hexuronic acid residues, β-d-mannuronopyranosyl (M) and α-l- guluronopyranosyl (G) residues. It can be arranged in the form of blocks of repeating M residues (MM blocks), blocks of repeating G residues (GG blocks), and blocks of mixed M and G residues (MG blocks). Commercially available alginate currently originates from algae. Alginate has wide applications. For example, one of its most important role is being used as wound dressing materials for the treatment of acute or chronic wounds. The use of alginate crosslinking to make hydrogels for cell encapsulation is also quite valuable. The emergence of various kinds of its derivatives recently has further extended its application.화학적 성질
Colorless or slightly yellow solid occurring in filamentous, granular, and powdered forms. Forms a viscous colloidal solution with water; insoluble in alcohol, ether, and chloroform. Combustible. Sodium alginate is mixed with a solution or suspension of the biocatalysts and then dropped into a calcium chloride solution to form water-insoluble calcium alginate gels that immobilize enzymes, cellular organelles, or microbial cells.물리적 성질
Sodium alginate occurs as an odorless and tasteless, white to pale yellowish-brown colored powder. insoluble in alcohol, ether or chloroform, etc. The aqueous solution of sodium alginate is stable at pH 4 to 10, and precipitates when pH < 3; hydrolysis occurs when pH > 10, and viscosity is lost at the same time.Characteristics
Sodium alginate [9005-38-3] is extracted from seaweed and is a linear copolymer of β-dmannuronic acid and α-l-guluronic acid linked by 1,4-glycosidic bonds. It forms a gel in the presence of multivalent ions, usually calcium or aluminum. The controlled entrapment of cells is simple and generally nontoxic. Various cell types can be immobilized with negligible loss of viability. However, the matrix can be solubilized in the presence of Ca2+-chelating agents such as phosphate, citrate, or ethylenediaminetetraacetic acid. The alginate matrix is mechanically weak so that the growing cells (especially plant cells) can be released from, or even disintegrate, the beads. Another drawback of the alginate method for viable animal cells is the difficulty of producing sufficiently small beads to overcome oxygen limitation in their interior.역사
Sodium alginate is a natural polysaccharide product that was first described in a patent application by the British chemist Edward C C Stanford in 1881. To this day brown algae are still the main source used to extract sodium alginate from. This group includes many of the seaweeds, like kelps, found in chilly northern seas. In addition to the food industry, the gelling properties of sodium alginate have been used in medical, dental and cosmetic applications for years.용도
- Sodium alginate can be used as a flavorless gum. It is used by the foods industry to increase viscosity and as an emulsifier. It is also used in indigestion tablets and the preparation of dental impressions.
- Sodium alginate (NaAlg) and its modified forms have been widely used as membranes in pervaporation (PV) separation of aqueous‐organic solutions because of the hydrophilic nature and versatility to modify/tune their structures to achieve the desired separation.
- Sodium alginate is a polymer which can be extracted from brown seaweed and kelps. It is one of the structural polymers that help to build the cell walls of these plants. It has some unusual properties and a wide variety of uses.
The polymer can be represented like this:
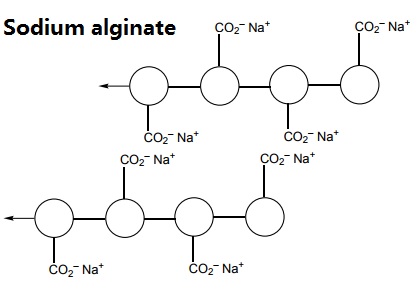
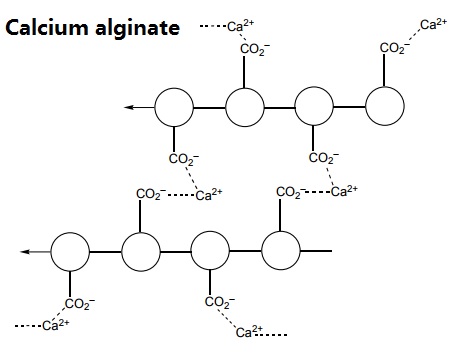
When sodium alginate is put into a solution of calcium ions, the calcium ions replace the sodium ions in the polymer. Each calcium ion can attach to two of the polymer strands. This is called cross-linking and can be represented like this:
주요 응용
Sodium Alginate is a gum obtained as a sodium salt of alginic acid, which is obtained from seaweed. it is coldand hot-water soluble, producing a range of viscosities. it forms irreversible gels with cal- cium salts or acids. it functions as a thickener, binder, and gelling agent in dessert gels, puddings, sauces, toppings, and edible films. In the manufacture of ice cream where it serves as a stabilizing colloid, insuring creamy texture and preventing the growth of ice crystals. In drilling muds; in coatings; in the flocculation of solids in water treatment; as sizing agent; thickener; emulsion stabilizer; suspending agent in soft drinks; in dental impression preparations. Pharmaceutic aid (suspending agent).생산 방법
Alginic acid is extracted from brown seaweed and is neutralized with sodium bicarbonate to form sodium alginate.일반 설명
Alginic acid sodium is a gelling and nontoxic anionic polysaccharide. The carboxylic acid groups on the alginic acid chain, renders it insoluble in water.However, converting alginic acid to its sodium form, enables it to solubilize in water easily.Safety Profile
Poison by intravenous and intraperitoneal routes. When heated to decomposition it emits toxic fumes of Na2OSafety
Sodium alginate is widely used in cosmetics, food products, and pharmaceutical formulations, such as tablets and topical products, including wound dressings. It is generally regarded as a nontoxic and nonirritant material, although excessive oral consumption may be harmful. A study in five healthy male volunteers fed a daily intake of 175 mg/kg body-weight of sodium alginate for 7 days, followed by a daily intake of 200 mg/kg body-weight of sodium alginate for a further 16 days, showed no significant adverse effects.TheWHOhas not specified an acceptable daily intake for alginic acid and alginate salts as the levels used in food do not represent a hazard to health.
Inhalation of alginate dust may be irritant and has been associated with industrial-related asthma in workers involved in alginate production. However, it appears that the cases of asthma were linked to exposure to seaweed dust rather than pure alginate dust.
LD50 (cat, IP): 0.25 g/kg
LD50 (mouse, IV): 0.2 g/kg
LD50 (rabbit, IV): 0.1 g/kg
LD50 (rat, IV): 1 g/kg
LD50 (rat, oral): >5 g/kg
저장
Sodium alginate is a hygroscopic material, although it is stable if stored at low relative humidities and a cool temperature.Aqueous solutions of sodium alginate are most stable at pH 4–10. Below pH 3, alginic acid is precipitated. A 1% w/v aqueous solution of sodium alginate exposed to differing temperatures had a viscosity 60–80% of its original value after storage for 2 years.) Solutions should not be stored in metal containers.
Sodium alginate solutions are susceptible on storage to microbial spoilage, which may affect solution viscosity. Solutions are ideally sterilized using ethylene oxide, although filtration using a 0.45 mm filter also has only a slight adverse effect on solution viscosity.
Heating sodium alginate solutions to temperatures above 70°C causes depolymerization with a subsequent loss of viscosity. Autoclaving of solutions can cause a decrease in viscosity, which may vary depending upon the nature of any other substances present. Gamma irradiation should not be used to sterilize sodium alginate solutions since this process severely reduces solution viscosity.
Preparations for external use may be preserved by the addition of 0.1% chlorocresol, 0.1% chloroxylenol, or parabens. If the medium is acidic, benzoic acid may also be used.
The bulk material should be stored in an airtight container in a cool, dry place.
Purification Methods
Free it from heavy metal impurities by treatment with ion-exchange resins (Na+-form), or with a dilute solution of the sodium salt of EDTA. Alternatively dissolve it in 0.1M NaCl, centrifuge and fractionally precipitate it by gradual addition of EtOH or 4M NaCl. The resulting gels are centrifuged off, washed with aqueous EtOH or acetone, and dried under vacuum. [Büchner et al. J Chem Soc 3974 1961.] Sodium n-alkylsulfates. Recrystallise these salts from EtOH/Me2CO [Hashimoto & Thomas J Am Chem Soc 107 4655 1985].비 호환성
Sodium alginate is incompatible with acridine derivatives, crystal violet, phenylmercuric acetate and nitrate, calcium salts, heavy metals, and ethanol in concentrations greater than 5%. Low concentrations of electrolytes cause an increase in viscosity but high electrolyte concentrations cause salting-out of sodium alginate; salting-out occurs if more than 4% of sodium chloride is present.Regulatory Status
GRAS listed. Accepted in Europe for use as a food additive. Included in the FDA Inactive Ingredients Database (oral suspensions and tablets). Included as an excipient in nonparenteral medicines (oral capsules, modified release tablets, enteric-coated tablets and lozenges) licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.참고 문헌
Pawar, Siddhesh N., and Kevin J. Edgar. Biomaterials 33.11 (2012): 3279-3305. Yang, Ji-Sheng, Ying-Jian Xie, and Wen He. Carbohydrate polymers 84.1 (2011): 33-39.알긴산나트륨 준비 용품 및 원자재
원자재
준비 용품
알긴산나트륨 공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Nanjing Sky Hope Tongyuan Biological Engineering Co., Ltd. | +86-0086-025-69916489 +86-18852044786 |
tongyuansales@vip.sina.com | China | 323 | 58 |
| Across Biotech Jinan Co LTD | +8613031735486 |
frank@acrossbiotech.com | China | 105 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 |
deasea125996@gmail.com | China | 2503 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 |
daisy@anhuiruihan.com | China | 994 | 58 |
| Hebei Jingbo New Material Technology Co., Ltd | +8619931165850 |
hbjbtech@163.com | China | 1000 | 58 |
| Shandong Juchuang Chemical Co., LTD | +undefined15030412209 |
admin@juchuangchem.com | China | 387 | 58 |
| airuikechemical co., ltd. | +undefined86-15315557071 |
sales02@airuikechemical.com | China | 994 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618740459177 |
sarah@tnjone.com | China | 941 | 58 |
| Ouhuang Engineering Materials (Hubei) Co., Ltd | +8617702722807 |
admin@hbouhuang.com | China | 2991 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 |
admin@zlchemi.com | China | 746 | 58 |





