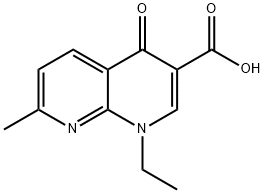
날리딕스산
|
|
날리딕스산 속성
- 녹는점
- 227-229 °C (lit.)
- 끓는 점
- 374.4°C (rough estimate)
- 밀도
- 1.2243 (rough estimate)
- 굴절률
- 1.6660 (estimate)
- 저장 조건
- 2-8°C
- 용해도
- 클로로포름 에 용해됨: 20mg/mL, 투명
- 물리적 상태
- 가루
- 산도 계수 (pKa)
- pKa 6.11± 0.02(Approximate)
- 색상
- 흰색에서 밝은 노란색
- 수용성
- 0.1G/L(23℃)
- Merck
- 14,6359
- BRN
- 750515
- 안정성
- 안정적인. 강한 산화제와 호환되지 않습니다.
- CAS 데이터베이스
- 389-08-2(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xn | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 63-42/43-40-20/21/22-22 | ||
| 안전지침서 | 22-36/37-45-24-36 | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | QN2885000 | ||
| F 고인화성물질 | 8-10-23 | ||
| HS 번호 | 29339190 | ||
| 유해 물질 데이터 | 389-08-2(Hazardous Substances Data) | ||
| 독성 | LD50 in mice (mg/kg): 3300 orally; 500 s.c.; 176 i.v. (Lesher, 1962) | ||
| 기존화학 물질 | KE-13602 |
날리딕스산 C화학적 특성, 용도, 생산
화학적 성질
Crystalline Powder용도
Nalidixic acid(NegGram) is a synthetic 1,8-naphthyridine antimicrobial agent with a limited bacteriocidal spectrum. It is an inhibitor of the A subunit of bacterial DNA gyrase. Evidence exists that the active metabolite, hydroxynalidixic acid, binds stron정의
ChEBI: A monocarboxylic acid comprising 1,8-naphthyridin-4-one substituted by carboxylic acid, ethyl and methyl groups at positions 3, 1, and 7, respectively.Antimicrobial activity
It displays good activity in vitro against a wide range of Enterobacteriaceae.일반 설명
Cream-colored powder.공기와 물의 반응
Insoluble in water.건강위험
SYMPTOMS: Ingestion of Nalidixic acid may cause nausea, vomiting, abdominal pain, allergic reactions and possible liver damage.화재위험
Flash point data for Nalidixic acid are not available, but Nalidixic acid is probably combustible.Pharmaceutical Applications
A 1,8 naphthyridone derivative available for oral administration.Pharmacokinetics
Oral absorption: >90%Cmax 1 g oral: c. 25 mg/L
Plasma half-life:c.1.5h
Volume of distribution :0.4 L/kg
Plasma protein binding: 93%
The plasma concentrations achieved after oral administration vary widely. In infants with acute shigellosis, absorption is much impaired by diarrhea. Administration with an alkaline compound leads to higher plasma concentrations, partly as the result of enhanced solubility (nalidixic acid is much more soluble at higher pH) and absorption and partly because of reduced tubular reabsorption.
It is rapidly metabolized, principally to the hydroxy acid, which is bacteriologically active, and glucuronide conjugates, which are not. The entire administered dose appears in the urine over a 24 h period. Elimination is reduced by probenecid. In the presence of renal impairment there is little accumulation of the active compound because it continues to be metabolized. However, elimination of metabolites is progressively delayed as renal function declines. About 4% of a dose appears in the feces.
Clinical Use
1-Ethyl-1,4-dihydro-7-methyl-4-oxo-1,8-naphthyridine-3-carboxylic acid (NegGram) occurs as a pale buff crystalline powder that is sparingly soluble in water and ether but solublein most polar organic solvents.Nalidixic acid is useful in the treatment of urinary tractinfections in which Gram-negative bacteria predominate.The activity against indole-positive Proteus spp. is particularlynoteworthy, and nalidixic acid and its congeners representimportant alternatives for the treatment of urinary tractinfections caused by strains of these bacteria resistant toother agents. Nalidixic acid is rapidly absorbed, extensivelymetabolized, and rapidly excreted after oral administration.The 7-hydroxymethyl metabolite is significantly more activethan the parent compound. Further metabolism of theactive metabolite to inactive glucuronide and 7-carboxylicacid metabolites also occurs. Nalidixic acid possesses at1/2elim of 6 to 7 hours. It is eliminated, in part, unchanged inthe urine and 80% as metabolites.부작용
Adverse reactions are generally those common to all quinolones: gastrointestinal tract and CNS disturbances and skin rashes, including eruptions related to photosensitivity. About half of the reported CNS reactions involve visual disturbances, hallucinations or disordered sensory perception. Severe excitatory states, including acute psychoses and convulsions, are usually observed in patients receiving high dosages. The drug should be avoided in patients with psychiatric disorders or epilepsy.Acute intracranial hypertension has been observed in children, some of whom have also manifested cranial nerve palsies. Hemorrhage has occurred in patients who were also receiving warfarin, presumably due to displacement of the anticoagulant from its protein binding sites by the nalidixic acid. Hemolytic anemia has been described several times in infants with or without glucose-6-phosphate dehydrogenase deficiency; in adults, death has occurred from autoimmune hemolytic anemia. Arthralgia and severe metabolic acidosis have rarely been reported.
Safety Profile
Poison by intravenous and intraperitoneal routes. Moderately toxic by ingestion and subcutaneous routes. An experimental teratogen. Human systemic effects: convulsions, hyperglycemia, sweating, and blood changes in children. Experimental reproductive effects.Questionable carcinogen with experimental carcinogenic and tumorigenic data. Human mutation data reported. Used as an antibacterial agent and urinary tract antiseptic. When heated to decomposition it emits toxic fumes of NOx.Purification Methods
Nalidixic acid crystallises from H2O or EtOH as a pale buff powder. It is soluble at 23o in CHCl3 (3.5%), toluene (0.16%), MeOH (0.13%), EtOH (0.09%), H2O (0.01%) and Et2O (0.01%). It inhibits nucleic acid and protein synthesis in yeast. [Lesher et al. J Med & Pharm Chem 5 1063 1962.]날리딕스산 준비 용품 및 원자재
원자재
Diethyl ester
2-Amino-5-methylpyridine
1,8-Naphthyridine
에톡시메틸렌말론산 디에틸에스테르
오소포름산에틸
5-Amino-2-methylpyridine
에틸브로마이드
말론산
요오드화에틸
수산화나트륨
2-Amino-6-methylpyridine
[N-Ethyl-N-(6-methyl-2-pyridyl)amino]methylenemalonic acid diethyl ester
ethyl 1-ethyl-1,4-dihydro-7-methyl-4-oxo-1,8-naphthyridine-3-carboxylate
ethyl 4-hydroxy-7-methyl-1,8-naphthyridine-3-carboxylate
diethyl [[(6-methyl-2-pyridyl)amino]methylene]malonate
준비 용품
날리딕스산 공급 업체
글로벌( 405)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 |
peter@yan-xi.com | China | 6000 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29797 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 |
info@dakenam.com | China | 15957 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21689 | 55 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 |
sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49391 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | 18192627656 |
1012@dideu.com | China | 2931 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 |
marketing@targetmol.com | United States | 19892 | 58 |
| Hubei Ipure Biology Co., Ltd | +8613367258412 |
ada@ipurechemical.com | China | 10326 | 58 |