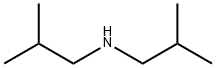
디이소부틸아민
|
|
디이소부틸아민 속성
- 녹는점
- -77 °C (lit.)
- 끓는 점
- 137-139 °C (lit.)
- 밀도
- 0.74 g/mL at 25 °C (lit.)
- 굴절률
- n
20/D 1.4081(lit.)
- 인화점
- 85 °F
- 저장 조건
- Flammables area
- 용해도
- insoluble to slightly soluble in water; soluble in ethanol, methanol, ethyl ether, ethyl acetate, acetone, benzene, aromatic and aliphatic hydrocarbons, fixed oils, mineral oil, oleic and stearic acids
- 산도 계수 (pKa)
- 11.07±0.28(Predicted)
- 물리적 상태
- 액체
- 색상
- 투명한
- 냄새
- 아민 냄새
- 수용성
- 5g/L(20℃)
- 감도
- Air Sensitive
- BRN
- 1209251
- Dielectric constant
- 2.7(22℃)
- LogP
- 2.383 (est)
- CAS 데이터베이스
- 110-96-3(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | C | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 10-22-34-52/53 | ||
| 안전지침서 | 26-36/37/39-45-25-16-61 | ||
| 유엔번호(UN No.) | UN 2361 3/PG 3 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | TX1750000 | ||
| F 고인화성물질 | 34 | ||
| TSCA | Yes | ||
| 위험 등급 | 3 | ||
| 포장분류 | III | ||
| HS 번호 | 29211990 | ||
| 유해 물질 데이터 | 110-96-3(Hazardous Substances Data) | ||
| 기존화학 물질 | KE-05-0500 |
디이소부틸아민 C화학적 특성, 용도, 생산
화학적 성질
The chemical reactivity of diisobutylamine is similar to other aliphatic amines and is governed by the unshared electron pair on the nitrogen atom. It is a strong base tending to form salts with acids. As with other secondary amines, diisobutylamine can be nitrosated, especially under acidic conditions, by nitrite ion or by nitrogen oxides from the air to form the carcinogenic and mutagenic N-nitrosodiisobutylamine (Olah et al 1975).용도
Diisobutylamine was used to study the effect of achiral amine on hydrogenation of ethyl pyruvate over cinchonidine-Pt/Al2O3 catalyst system. Diisobutylamine is the principal starting material for the herbicide Butylate (Zeneca).생산 방법
Diisobutylamine can be produced by the reaction of ammonia and butanol over a dehydration catalyst at high temperature and pressure (Hawley 1977). Alternatively, ammonia, butanol, and hydrogen can be passed over a dehydrogenation catalyst. In 1976, 18,000 tons of diisobutylamine were produced (Schweizer et al 1978). Diisobutylamine is also naturally present in foods and soil.As with other secondary amines, diisobutylamine can be nitrosated to form the highly toxic (Olah 1975) N-nitrosodiisobutylamine (Guttenplan 1987; Vlasenko et al 1981; Spiegeholder et al 1978). Thus, nitrosation of commercial preparations of diisobutylamine occurs on standing, presumably by reaction with nitrogen oxides in the air (Spiegelhalder et al 1978) and N-nitrosodiisobutylamine has been found in various fishery products (Kawabata et al 1974) and other foods (Osborne 1972; Telling 1972).
일반 설명
Diisobutylamine appears as a clear colorless liquid with an ammonia-like odor. Insoluble in water and less dense than water. Hence floats on water. Vapors heavier than air. Toxic oxides of nitrogen produced during combustion.공기와 물의 반응
Highly flammable. Sensitive to heat and air. Insoluble in water.반응 프로필
Diisobutylamine can react vigorously with oxidizing materials . Neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides.건강위험
Diisobutylamine is a relatively strong base and is therefore irritating to the eyes, respiratory tract, and skin. Inhalation of vapors can result in pulmonary edema following prolonged exposure. Ingestion of liquid can cause severe burning of the esophagus.공업 용도
Diisobutylamine is used as a chemical intermediate in the manufacture of several agricultural and pharmaceutical products.Safety Profile
Poison by ingestion. A dangerous fire hazard when exposed to heator flame; can react vigorously with oxidizing materials. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits toxic fumes of Nox신진 대사
There is little information available on the metabolism of diisobutylamine.디이소부틸아민 준비 용품 및 원자재
원자재
준비 용품
디이소부틸아민 공급 업체
글로벌( 206)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12470 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 |
deasea125996@gmail.com | China | 2503 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21689 | 55 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 |
sales@sdzschem.com | China | 2931 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 |
alice@crovellbio.com | China | 8822 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 |
sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49391 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; |
factory@coreychem.com | China | 29826 | 58 |
디이소부틸아민 관련 검색:
아이소부탄 모노부틸아민 다이-n-뷰틸아민 이소부틸아민 디이소부틸아민 염화수소 2,2'-아조비스-(2-아미노프로판)이염산염 디-2-에틸헥실아민 디이소부틸아민 2-에틸-N,N-비스(2-에틸헥실)
Hexetidine
SOLASODINE
SOLASONINE
VERATRINE
TOMATINE
Octyl(phenyl)-N,N-diisobutylcarbamoylmethylphosphine oxide
2-Chloro-N,N-bis(2-methylpropyl)acetamide
TRIISOBUTYLAMINE
N,N-DIISOBUTYLETHYLENEDIAMINE