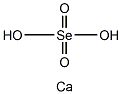
칼슘 셀레네이트
|
|
칼슘 셀레네이트 속성
- Solubility Product Constant (Ksp)
- pKsp: 3.09
안전
| 기존화학 물질 | KE-04605 | ||
|---|---|---|---|
| 유해화학물질 필터링 | 97-1-134 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 셀레늄 또는 그 화합물과 셀렌화합물을 1% 이상 함유한 혼합물 |
칼슘 셀레네이트 C화학적 특성, 용도, 생산
개요
Calcium selanate has the molecular formula of CaSeO4 and the molecular weight of 201.0598 g/mol. It can be prepared by reacting sodium selenate with calcium chloride in solution:CaCl2(aq)+ Na2SeO4(aq) ? CaSeO4+ 2NaCl(aq)
The result is calcium selenate dihydrate CaSe- O4·2H2O whose CAS number is 7790-74-1. It is slightly soluble in water at 9.22 g/100 ml at 20°C.
Evaporating the solution serves to form white, monoclinic crystals whose molecular weight is 219.07 g/mol, with a density of 2.75 g/cm3.
용도
Amajor use for calciumselenate has been the formation of CaSe by reduction with hydrogen gas. This salt is readily available commercially by several manufacturers。일반 설명
White powder.공기와 물의 반응
Water soluble반응 프로필
Inorganic oxidizing agents can react with reducing agents to generate heat and products that may be gaseous (causing pressurization of closed containers). The products may themselves be capable of further reactions (such as combustion in the air). The chemical reduction of materials in this group can be rapid or even explosive, but often requires initiation (heat, spark, catalyst, addition of a solvent). Explosive mixtures of inorganic oxidizing agents with reducing agents often persist unchanged for long periods if initiation is prevented. Such systems are typically mixtures of solids, but may involve any combination of physical states. Some inorganic oxidizing agents are salts of metals that are soluble in water; dissolution dilutes but does not nullify the oxidizing power of such materials. Organic compounds in general have some reducing power and can in principle react with compounds in this class. Actual reactivity varies greatly with the identity of the organic compound. Inorganic oxidizing agents can react violently with active metals, cyanides, esters, and thiocyanates. Explosives often consist of an inorganic oxidizing agent mixed in intimate contact with a reducing agent. Gunpowder is such a mixture. Other examples are a mixture of sugar (an organic compound) plus sodium chlorate and magnesium (an inorganic reducing agent) plus barium peroxide.건강위험
Highly toxic, may be fatal if inhaled, swallowed or absorbed through skin. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.화재위험
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Containers may explode when heated. Runoff may pollute waterways.칼슘 셀레네이트 준비 용품 및 원자재
원자재
준비 용품
칼슘 셀레네이트 공급 업체
글로벌( 11)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| 3B Scientific Corporation | -- |
sales@3bsc.com | United States | 6971 | 56 |
| Leancare Ltd. | -- |
enquiry@leancare.co.uk | United Kingdom | 6460 | 42 |
| 2A PharmaChem USA | -- |
sales@2apharmachem.com | United States | 6148 | 39 |
| 3B Scientific Corporation | -- |
sales@3bsc.com | United States | 6744 | 47 |
| HONEST JOY HOLDINGS LIMITED | -- |
sales@honestjoy.cn | United States | 6702 | 54 |
| City Chemical LLC | -- |
sales@citychemical.com | United States | 6710 | 72 |
| Service Chemical Inc. | -- |
sales@chemos-group.com | Germany | 6373 | 71 |
| Pfaltz & Bauer, Inc. | -- |
sales@pfaltzandbauer.com | United States | 6830 | 72 |
| City Chemicals Corporation | -- |
United States | 6462 | 72 | |
| Advanced Technology & Industrial Co., Ltd. | -- |
sales@advtechind.com | China | 6531 | 75 |