테트라민 C화학적 특성, 용도, 생산
개요
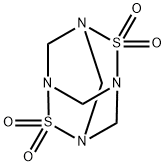
Tetramethylenedisulfotetramine (2,6-dithia-1,3,5,7-tetraazadamantane,
2,2,6,6-tetraoxide, TETS) is a highly toxic heteroadamantane
rodenticide. It is an odorless, tasteless, white
crystalline powder that is slightly soluble in water
(0.25 mg ml
-1), dimethyl sulfoxide and acetone. It was originally
synthesized in 1933 as a resinous condensation product
of sulfamide and formaldehyde and used commercially in
pillows and upholstery as an impregnating stiffening and antimold
agent. However, in 1950, a massive poisoning of
German workers in the furniture manufacturing industry was
linked to ‘Crinex’ wool, which contained TETS as a byproduct
of processing. Early experimental studies in rodents revealed
that TETS was an extremely toxic convulsant agent. It was also
discovered at this time that TETS is a highly effective rodent
repellent, which resulted in its use during reforestation
projects to prevent seed predation by rodents. However,
because of its high toxicity in mammals, including humans,
and its persistence in the environment, many countries banned
its production and use in 1984. This ban became worldwide
when China issued similar restrictions in 1991. However,
due to its relative ease of synthesis and low cost, TETS remains
available on the black market, particularly in many rural areas
of China and in regions outside of China that have large Asian
populations.
용도
Despite the worldwide ban on its production and use, TETS
continues to be used illicitly as a rodenticide in various regions
of the world. In China, TETS is known as ‘Dushuqiang’,
‘Meishuming,’ or ‘Shanbudao.’ In 2000, the National Poison
Control Center of China revealed that 74% of commercial
rodenticides contained illegal chemicals, with TETS found in nearly 50% of these pesticides. From 1977 to 2002, it was
estimated that there were thousands of cases of TETS poisoning
in China, resulting in hundreds of deaths. A more recent
analysis indicates that between 1991 and 2010, there were over
14 000 cases of TETS intoxication in China, of which 932
resulted in death. In 2003, the first case of TETS intoxication in
the United States was reported: a healthy 15-month-old girl
was poisoned following accidental ingestion of a rodenticide
imported from China that contained TETS. While many cases
are thought to be due to accidental poisonings, there have been
numerous reports of TETS being used to intentionally poison
humans.
환경귀착
Although TETS has a relatively low solubility in water
(0.25 mg kg
-1), it is quite stable, thus making it relatively
persistent in the environment. It is reported that TETS retains
biological activity in water for 6 weeks to 5 months after preparation.
It is believed that TETS bioaccumulates (despite a predicted
octanol:water coefficient of 0.07) and that contact with
poisoned animals can result in intoxication, as demonstrated by
reports of dogs dying after eating TETS-poisoned rats and by
Chinese newspapers warning against consuming meat from
dogs that were suspected to have eaten TETS-poisoned rats.
테트라민 준비 용품 및 원자재
원자재
준비 용품