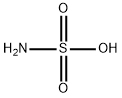
설파믹산
|
|
설파믹산 속성
- 녹는점
- 215-225 °C (dec.) (lit.)
- 끓는 점
- -520.47°C (estimate)
- 밀도
- 2.151 g/cm3 at 25 °C
- 증기압
- 0.8Pa at 20℃
- 굴절률
- 1.553
- 저장 조건
- Store below +30°C.
- 용해도
- 물: 20°C에서 용해성213g/L
- 산도 계수 (pKa)
- -8.53±0.27(Predicted)
- 물리적 상태
- 결정 또는 결정성 분말
- 색상
- 하얀색
- 수소이온지수(pH)
- 1.2 (10g/l, H2O)
- 냄새
- 냄새 없는
- 수용성
- 146.8g/L(20℃)
- Merck
- 14,8921
- 안정성
- 안정적인.
- InChIKey
- IIACRCGMVDHOTQ-UHFFFAOYSA-N
- LogP
- 0 at 20℃
- CAS 데이터베이스
- 5329-14-6(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 36/38-52/53 | ||
| 안전지침서 | 26-28-61-28A | ||
| 유엔번호(UN No.) | UN 2967 8/PG 3 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | WO5950000 | ||
| TSCA | Yes | ||
| 위험 등급 | 8 | ||
| 포장분류 | III | ||
| HS 번호 | 28111980 | ||
| 유해 물질 데이터 | 5329-14-6(Hazardous Substances Data) | ||
| 독성 | MLD orally in rats: 1.6 g/kg (Ambrose) | ||
| 기존화학 물질 | KE-32336 |
설파믹산 C화학적 특성, 용도, 생산
용도
표백, 단마제의 원료, 아질산 이온제거제,금소표면의세척,유기합성에 사용.제품특징
- 비휘발성이며 비흡습성의 백색의 결정체.- 물에 녹을 때 강한 산이된다.
- 물, 암모니아수, 피리딘 등에 녹으며 알콜에는 약용해성 에테르에는 불용성이다.
- 건조한 냉암소에 밀봉화여 산화성물질과 분리시켜 저장한다.
- 세척제, 세정제, 청관제, 도료, 동도금 제조액, pH조절제, 표백, 단마제의 원료, 아질산 이온제거제 등에 사용된다.
제품용도
- 표백- 염료
- 도금약품
- 청관제
- 피혁용 세척제
- 브라운관 공정촉매
화학적 성질
Sulfamic acid is a white orthorhombic flaky crystal, odorless, non-volatile and non-hygroscopic. Soluble in water and liquid ammonia, slightly soluble in methanol, insoluble in ethanol and ether, also insoluble in carbon disulfide and liquid sulfur dioxide. Its aqueous solution has the same strong acid properties as hydrochloric acid and sulfuric acid, but its corrosiveness to metals is much lower than that of hydrochloric acid. The toxicity is extremely small, but it should not be in contact with the skin for a long time, and it should not enter the eyes.용도
Sulfamic acid is widely used in electroplating, hard-water scale reremovers, acidic cleaning agent, chlorine stabilizers, sulfonating agents, denitrification agents, disinfectants, flame retardants, herbicides, artificial sweeteners and catalysts.Sulfamic acid is a precursor to sweet-tasting compounds. Reaction with cyclohexylamine followed by addition of NaOH gives C6H11NHSO3Na, sodium cyclamate.
Sulfamic acid is a water-soluble, moderately strong acid. An intermediate between sulfuric acid and sulfamide, it can be used as a precursor to sweet-tasting compounds, a therapeutic drug component, an acidic cleaning agent, and a catalyst for esterification.
정의
ChEBI: Sulfamic acid is the simplest of the sulfamic acids consisting of a single sulfur atom covalently bound by single bonds to hydroxy and amino groups and by double bonds to two oxygen atoms. It is a strong acid, readily forming sulphamate salts, which is extremely soluble in water and normally exists as the zwitterion H3N+. SO3–.주요 응용
Sulfamic acid, the monoamide of sulfuric acid, is a strong inorganic acid. It is generally used in chemical cleaning processes like removal of nitrites, carbonate- and phosphate-containing deposits.Sulfamic acid can be used as a catalyst in:
Friedlander quinoline synthesis.
Liquid Beckmann rearrangement for the synthesis of amides from ketoximes.
The preparation of α-aminophosphonates via a three-component reaction between aldehydes, amines, and diethyl phosphite.
화학 반응
Sulfamic acid is a strong acid that reacts with many basic compounds. It is heated to above the melting point (209°C) under normal pressure to begin to decompose, and continues to be heated to above 260°C to decompose into sulfur trioxide, sulfur dioxide, nitrogen, hydrogen and water.(1) Sulfamic acid can react with metals to form transparent crystalline salts. Such as:
2H2NSO3H+Zn→Zn(SO3NH2)2+H2.
(2) Can react with metal oxides, carbonates and hydroxides:
FeO+2HSO3NH2→Fe(SO3NH2)2+H2O2
CaCO3+2HSO3NH2→Ca(SO3NH2)2+H2O+CO23
Ni(OH)2+2HSO3NH2→Ni(SO3NH2)2+H2O.
(3) Can react with nitrate or nitrite:
HNO3+HSO3NH2→H2SO4+N2O+H2O2
HNO2+HSO3NH2→H2SO4+N2+H2O.
(4) Can react with oxidants (such as potassium chlorate, hypochlorous acid, etc.):
KClO3+2HSO3NH2→2H2SO4+KCl+N2+H2O2
2HOCl+HSO3NH2→HSO3NCl2+2H2O
일반 설명
Sulfamic acid appears as a white crystalline solid. Density 2.1 g / cm3. Melting point 205°C. Combustible. Irritates skin, eyes, and mucous membranes. Low toxicity. Used to make dyes and other chemicals. It is used as a raw material for the preparation of a synthetic sweetener i.e, sodium cyclohexylsulfamate.공기와 물의 반응
Moderately soluble in water [Hawley].반응 프로필
Sulfamic acid reacts exothermically with bases. Aqueous solutions are acidic and corrosive.위험도
Toxic by ingestion.건강위험
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.화재위험
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.Safety Profile
Poison by intraperitoneal route. Moderately toxic by ingestion. A human skin irritant. A corrosive irritant to skin, eyes, and mucous membranes. A substance that migrates to food from packaging materials. Violent or explosive reactions with chlorine, metal nitrates + heat, metal nitrites + heat, fuming HNO3. When heated to decomposition it emits very toxic fumes of SOx and NOx. See also SULFONATES.Toxicology
Sulfamic acid can be absorbed into the body through inhalation of its aerosols and ingestion, causing symptoms of coughing and sore throat upon inhalation; it is irritating to the skin and eyes with symptoms of redness, pain or burns; Inhaling sulfamic acid can irritate the nose and throat. High exposure to sulfamic acid can irritate the lungs. Higher exposures may cause a build-up of fluid in the lungs (pulmonary edema), a medical emergency. sulfamic acid can cause nausea, vomiting and stomach pain. Repeated exposure to sulfamic acid may affect the liver and kidneys.잠재적 노출
Sulfamic acid is used in metal and ceramic cleaning, bleaching paper pulp; and textiles metal; in acid cleaning; as a stabilizing agent for chlorine and hypochlorite in swimming pools; cooling towers; and paper mills.운송 방법
UN2967 Sulfamic acid, Hazard class: 8; Labels: 8-Corrosive material.Purification Methods
Crystallise NH2SO3H from water at 70o (300mL per 25g), after filtering, by cooling a little and discarding the first batch of crystals (about 2.5g) before standing in an ice-salt mixture for 20minutes. The crystals are filtered off by suction, washed with a small quantity of ice cold water, then twice with cold EtOH and finally with Et2O. Dry it in air for 1hour, then store it in a desiccator over Mg(ClO4)2 [Butler et al. Ind Eng Chem (Anal Ed) 10 690 1938]. For the preparation of primary standard material see Pure Appl Chem 25 459 1969.비 호환성
The aqueous solution is a strong acid. Reacts violently with strong acids (especially fuming nitric acid), bases, chlorine. Reacts slowly with water, forming ammonium bisulfate. Incompatible with ammonia, amines, isocyanates, alkylene oxides; epichlorohydrin, oxidizers.설파믹산 준비 용품 및 원자재
원자재
준비 용품
sodium nonylphenol polyoxyethylene ether sulfate
설파민산니켄
적색 194 분산염료
fatty alcohol ammonium sulfate
Acesulfame
AMIDO GREEN BLACK B
락톤
메틸 오렌지
벤설프론 메틸
Pigment Red 175
포타슘아세설팜
3,5-다이브로모-4-하이드록시벤조나이트릴 및 그 염류
설파믹산암모늄
SULCOFURON-SODIUM MONOHYDRATE
nonylphenyl polyoxyethylene ether sulfate triethanolamine
코발트 술펌산
Vadimezan
Direct Black 56
Filth remover
2-Chlorobenzonitrile
Irsogladine
소듐사이클라메이트
당밀
Cleaning agent
METOSULAM
Disperse Yellow 104
설파믹산 공급 업체
글로벌( 542)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Qingdao Trust Agri Chemical Co.,Ltd | +8613573296305 |
aroma@qdtrustagri.com | China | 165 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 |
sales@tnjchem.com | China | 34572 | 58 |
| Wuhan Bright Chemical Co., Ltd | +86-02785804899 +86-15071395570 |
alice@brightchemical.com.cn | China | 93 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
fandachem@gmail.com | China | 9347 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 |
info@tnjchem.com | China | 2989 | 55 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 |
sales@sdzschem.com | China | 2931 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Jinan Finer Chemical Co., Ltd | +86-531-88989536 +86-15508631887 |
sales@finerchem.com | China | 2968 | 58 |