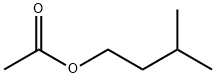
초산 이소아밀
|
|
초산 이소아밀 속성
- 녹는점
- -78 °C (lit.)
- 끓는 점
- 142 °C/756 mmHg (lit.)
- 밀도
- 0.876 g/mL at 25 °C (lit.)
- 증기 밀도
- 4.5 (vs air)
- 증기압
- 5 mm Hg ( 25 °C)
- 굴절률
- n
20/D 1.4(lit.)
- 인화점
- 77 °F
- 저장 조건
- Store at +5°C to +30°C.
- 용해도
- 에탄올: 용해성1ml/3ml, 투명, 무색(60%에탄올)
- 색상
- 무색의
- 냄새
- 바나나 같은 냄새
- 폭발한계
- 1-10%(V)
- ?? ??
- 과일 같은
- 수용성
- 0.20g/100mL. 약간 용해됨
- Merck
- 14,5111
- JECFA Number
- 43
- BRN
- 1744750
- Henry's Law Constant
- 10.25 at 37 °C (static headspace-GC, Bylaite et al., 2004)
- 노출 한도
- TLV-TWA 100 ppm (~530 mg/m3) (ACGIH, MSHA, and OSHA); TLV-STEL 125 ppm (~655 mg/m3); IDLH 3000 ppm (NIOSH).
- Dielectric constant
- 5.6(20℃)
- LogP
- 2.7 at 35℃
- CAS 데이터베이스
- 123-92-2(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 10-66-36/37/38-R66-R10 | ||
| 안전지침서 | 23-25-2-36/37/39-26-16-S25-S23-S2 | ||
| 유엔번호(UN No.) | UN 1104 3/PG 3 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | NS9800000 | ||
| 자연 발화 온도 | 680 °F | ||
| TSCA | Yes | ||
| 위험 등급 | 3 | ||
| 포장분류 | III | ||
| HS 번호 | 29153900 | ||
| 유해 물질 데이터 | 123-92-2(Hazardous Substances Data) | ||
| 독성 | LD50 orally in Rabbit: > 5000 mg/kg LD50 dermal Rat > 5000 mg/kg | ||
| IDLA | 1,000 ppm | ||
| 기존화학 물질 | KE-23580 |
초산 이소아밀 C화학적 특성, 용도, 생산
순도시험
(1) 비중 : 이 품목의 비중은 0.868~0.878이어야 한다.
(2) 굴절률 : 이 품목의 굴절률 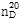 은 1.400~1.404이어야 한다.
은 1.400~1.404이어야 한다.
(3) 용상 : 이 품목 1mL를 60% 에탄올 3mL에 녹일 때, 그 액은 징명하여야 한다.
(4) 산가 : 이 품목의 산가는 향료시험법 중 산가측정법에 따라 시험할 때, 1 이하이어야 한다.
확인시험
이 품목 1mL에 10% 알콜성수산화칼륨용액 5mL를 넣어 수욕중에서 흔들어 섞으면서 가열하면 특이한 향기는 없어지고 이소아밀알콜냄새가 남는다. 식힌 다음 이에 물 10mL 및 묽은염산 0.5mL를 넣은 액은 확인시험법 중 초산염 (다)의 반응을 나타낸다 .
정량법
이 품목 약 0.5g을 정밀히 달아 향료시험법 중 에스테르가 및 에스테르함량측정법에 따라 시험한다.
0.5N 알콜성수산화칼륨용액 1mL = 65.09mg C7H14O2
개요
In commercial practice amyl invariably means isoamyl, unless it is prefaced by the n- for normal. Isoamyl acetate has a powerful, fruity odor with a bittersweet taste reminiscent of pear. If impure, the odor is strong, penetrating, and almost shocking. Usually prepared by esterification of commercial isoamyl alcohol with acetic acid.화학적 성질
Isoamyl Acetate is a strongly fruity-smelling liquid and has been identified in many fruit aromas. It is the main component of banana aroma and is, therefore, also used in banana flavors.All isomers of amyl acetate are highly flammable, colorless to yellow, watery liquids.
Isoamyl acetate has a fruity, banana, sweet, fragrant, powerful odor with a bittersweet taste reminiscent of pear. If impure, the odor is strong, penetrating and almost shocking. In commercial practice, amyl invariably means isoamyl.
물리적 성질
Clear, colorless liquid with a banana or pear-like odor. Odor threshold concentration is 7 ppm (quoted, Keith and Walters, 1992). A detection odor threshold concentration of 18 μg/m3 (3.4 ppbv) was determined by Katz and Talbert (1930).출처
Reported to be found in a number of naturally occurring products, including apple, banana, cocoa bean, coffee, cognac, grape, peach, pear, pineapple and strawberry용도
Isoamyl acetate is used to impart pear flavorto mineral waters and syrups, in perfumes, inthe manufacture of artificial silk or leather, inphotographic films, in dyeing textiles, and asa solvent.정의
ChEBI: The acetate ester of isoamylol.제조 방법
By the esterification of commercial isoamyl alcohol with acetic acid생산 방법
The commerical-grade isoamyl acetate is prepared by the esterification of amyl alcohol (often fusel oil) with acetic acid and a small amount of sulfuric acid as the catalyst .일반 설명
Oily liquid; colorless; banana odor. Floats and mixes with water. Flammable, irritating vapor is produced .공기와 물의 반응
Highly flammable. Insoluble in water.반응 프로필
Isoamyl acetate is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. Isoamyl acetate can react violently with oxidizing materials, nitrates, strong alkalis and strong acids.위험도
Flammable, moderate fire risk. Irritant. Explosive limits in air 1–7.5%.건강위험
Isoamyl acetate exhibits low toxicity; thetoxic effects are comparable to those of n amyl acetate. The toxic symptoms includeirritation of the eyes, nose, and throat;fatigue; increased pulse rate; and narcosis.Inhalation of its vapors at 1000 ppm for30 minutes may cause irritation, fatigue, andrespiratory distress in humans. It is more narcotic than are the lower acetic esters. TheLD50 value in rabbits is on the order of7000 mg/kg.화재위험
FLAMMABLE. Flashback along vapor trail may occur. Vapor may explode if ignited in an enclosed area. When heated emits acrid fumes. When exposed to flames can react vigorously with reducing materials.잠재적 노출
(n-isomer): Primary irritant (w/o allergic reaction), (sec-isomer) Human Data. Amyl acetates are used as industrial solvents and in the manufacturing and dry-cleaning industry; making artificial fruit-flavoring agents; cements, coated papers, lacquers; in medications as an inflammatory agent; pet repellents, insecticides and miticide. Many other uses.Carcinogenicity
Not listed by ACGIH, California Proposition 65, IARC, NTP, or OSHA.환경귀착
Chemical/Physical. Slowly hydrolyzes in water forming 3-methyl-1-butanol and acetic acid.운송 방법
UN1993 Flammable liquids, n.o.s., Hazard Class: 3; Labels: 3-Flammable liquid, Technical Name Required.Purification Methods
Dry the acetate with finely divided K2CO3 and fractionally distil it. [Beilstein 2 IV 157.]비 호환성
Vapors may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, nitrates. May soften certain plastics.폐기물 처리
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.초산 이소아밀 준비 용품 및 원자재
원자재
준비 용품
초산 이소아밀 공급 업체
글로벌( 515)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Henan Xiangduo Industry Co., Ltd. | +86-15981848961 +86-15981848961 |
sales@xiangduochem.com | China | 497 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 |
deasea125996@gmail.com | China | 2503 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 |
info@tnjchem.com | China | 2989 | 55 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 |
alice@crovellbio.com | China | 8823 | 58 |
| Cangzhou Wanyou New Material Technology Co.,Ltd | 18631714998 |
sales@czwytech.com | CHINA | 906 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 |
sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |