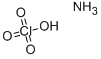과염소산암모늄
|
|
과염소산암모늄 속성
- 녹는점
- °Cd ec.)
- 밀도
- 1.95 g/mL at 25 °C(lit.)
- 증기압
- 0Pa at 25℃
- 굴절률
- 1.482
- 용해도
- soluble in methanol; slightly soluble in ethanol, acetone; insoluble in ethyl ether
- 물리적 상태
- 수정 같은
- Specific Gravity
- 1.95
- 색상
- 하얀색
- 수용성
- 물에 자유롭게 용해됩니다. 메탄올에 용해됩니다. 에탄올, 아세톤에 약간 용해됩니다. 에테르, 에틸 아세테이트에 불용성
- Merck
- 14,540
- 안정성
- 가연성 물질과 혼합되면 폭발성이 있습니다. 유기물, 종이, 나무 부스러기 등과 호환되지 않습니다. 밀폐된 상태에서 가열하거나 마찰로 인해 폭발할 수 있습니다. 금속, 환원제, 강산과 호환되지 않습니다.
- LogP
- -5.84 at 25℃
- CAS 데이터베이스
- 7790-98-9(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | O,Xi,Xn,E | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 9-44-48/20/22-36-3 | ||
| 안전지침서 | 17-36/37-27-16-14-35-26 | ||
| 유엔번호(UN No.) | 1442 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | SC7520000 | ||
| TSCA | Yes | ||
| 위험 등급 | 5.1 | ||
| 포장분류 | II | ||
| 유해 물질 데이터 | 7790-98-9(Hazardous Substances Data) | ||
| 독성 | LD50 oral in rabbit: 1900mg/kg | ||
| 기존화학 물질 | KE-01725 |
과염소산암모늄 C화학적 특성, 용도, 생산
개요
Ammonium perchlorate is also shock-sensitive and may explode when exposed to heat or by spontaneous chemical reaction. This is the material that was involved in the explosion at the Pepcon plant in Henderson, Nevada. It is also used in the production of explosives, pyrotechnics, etching and engraving, and jet and rocket propellants.화학적 성질
Ammonium perchlorate NH4CI04, is a white solid, soluble, formed by reaction of NH40H and perchlorate acid, and then evaporating. Used in explosives and pyrotechnics.용도
Perchlorate is a soluble oxychloro anion most commonly used as a solid salt in the form of ammonium perchlorate, potassium perchlorate, lithium perchlorate, or sodium perchlorate, all of which are highly soluble. Ammonium perchlorate is the most widely used perchlorate compound. In their pure forms, these salts are white or colorless crystals or powders. Perchlorate salts dissolve in water and readily move from surface to groundwater. Perchlorate is known to originate from both natural and man-made sources.The most common uses for ammonium perchlorate are in explosives, military munitions, and rocket propellants. In addition, perchlorate salts are used in a wide range of nonmilitary applications, including pyrotechnics and fireworks, blasting agents, solid rocket fuel, matches, lubricating oils, nuclear reactors, air bags, and certain types of fertilizers.
일반 설명
A white, crystalline solid or powder. Classified as a division 1.1 explosive if powdered into particles smaller than 15 microns in diameter or if powdered into larger particles but thoroughly dried. Does not readily burn, but will burn if contaminated by combustible material. May explode under prolonged exposure to heat or fire. Used to make rocket propellants, explosives, pyrotechnics, as an etching and engraving agent, and in analytical chemistry.공기와 물의 반응
Water soluble.반응 프로필
AMMONIUM PERCHLORATE is a strong oxidizing agent. Decomposes at 130°C and explodes at 380°C [Mellor 2 Supp. 1:608 1956]. Explosions have occurred in propellant formulations containing AMMONIUM PERCHLORATE to which ferrocene has been added as a burning rate catalyst. Although the cause was not been definitely established, AMMONIUM PERCHLORATE was most probably frictional heating from dragging a spatula through the mixture [ASESB Expl. Report 211 1966]. Can explode when mixed with sugar, charcoal or on contact with hot copper pipes. Becomes impact-sensitive when contaminated by powdered carbon, ferrocene, sulfur, or other reducing materials such as organic matter or powdered metals.위험도
Strong oxidizing agent; ignites violently with combustibles. Shock sensitive; may explode when exposed to heat or by spontaneous chemical reaction. Sensitive, high explosive when contaminated with reducing materials. Skin irritant.건강위험
Irritating to skin and mucous membranes.Safety Profile
moderately toxic by ingestion and parented routes. Flammable when exposed to heat or flame or by spontaneous chemical reaction with reducing materials. A very powerful oxidizer that has caused explosions in industry. Ignites violently with combustibles. Severe explosion hazard; decomposes at 130' and explodes at 380'. When contaminated by powdered carbon, ferrocene, S, organic matter, powdered metals, nitryl perchlorate, potassium periodate, potassium permanganate, it becomes impact sensitive. Potentially explosive reactions with carbon (above 240℃), dichromium trioxide (at 270℃), cadmium oxide (at 260℃), zinc oxide (at 200°C), copper chromite, copper oxide, iron oxide, potassium permanganate, potassium dichromate, mono-, di-, tri-, or tetra-methylammonium perchlorates, metal perchlorates (e.g., lithium perchlorate, zinc perchlorate), nitrophenol-formaldehyde polymer. Mixtures with aluminum or copper burn violently when ignited. Mixtures with ethylene dinitrate ignite when stored at 60℃. When heated to decomposition it emits toxic fumes of NH3 and Cl-. See also PERCHLORATES and EXPLOSIVES, HIGH.Purification Methods
It is recrystallised twice from distilled water (2.5mL/g) between 80o and 0o, and dried in a vacuum desiccator over P2O5. Drying at 110o might lead to slow decomposition to the chloride. POTENTIALLY EXPLOSIVE.과염소산암모늄 준비 용품 및 원자재
원자재
준비 용품
과염소산암모늄 관련 검색:
염소산 암모늄 과염소산 과염소산암모늄 하이드록실암모늄 과염소산염 크레실 바이올렛 퍼클로레이트
TETRAPROPYLAMMONIUM PERCHLORATE
RHODAMINE 6G PERCHLORATE
OXAZINE 1
TETRAETHYLAMMONIUM PERCHLORATE
Tetrabutylammonium perchlorate
IR-140
TETRA-N-HEXYLAMMONIUM PERCHLORATE
dimethyl{4-[1,5,5-tris(4-dimethylaminophenyl)-2,4-pentadienylidene]-2,5-cyclohexadien-1-ylidene}ammonium perchlorate
TETRAMETHYLAMMONIUM PERCHLORATE
3,3'-DIETHYLTHIATRICARBOCYANINE PERCHLORATE
N-TERT-BUTYL-5-METHYLISOXAZOLIUM PERCHLORATE
DIMETHYLAMINONAPHTHALENE-5-SULFONAMIDO-ETHYLTRIMETHYL AMMONIUM PERCHLORATE,DASP Dimethylaminonaphthalene-5-sulfonamidoethyltrimethyl ammonium perchlorate
ACETYLCHOLINE PERCHLORATE