Zinc sulphate
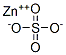
|
- $7 - $189
- Product name: Zinc sulphate
- CAS: 7733-02-0
- MF: ZnSO4
- MW: 161.45
- EINECS:231-793-3
- MDL Number:MFCD00011302
- Synonyms:ai3-03967 ;Bonazen;Bufopto zinc sulfate;ZincSulphate,>90%;ZincSulphate,>98%;zinc sulfate solution;ZINC(II)SULPHATE;Zinksulfat
10 prices
Selected condition:
Brand
- Alfa Aesar
- BLDpharm
- Sigma-Aldrich
- TRC
Package
- 5g
- 10g
- 25g
- 100ml
- 250ML
- 500ml
- 1L
- 1amp
- ManufacturerAlfa Aesar
- Product numberJ60963
- Product descriptionZinc sulfate 100mM aq. soln.
- Packaging100ml
- Price$60.7
- Updated2023-06-20
- Buy
- ManufacturerBLDpharm
- Product numberBD157254
- Product descriptionZinc(II)sulfate 99%
- Packaging5g
- Price$7
- Updated2021-12-16
- Buy
- ManufacturerBLDpharm
- Product numberBD157254
- Product descriptionZinc(II)sulfate 99%
- Packaging10g
- Price$10
- Updated2021-12-16
- Buy
- ManufacturerBLDpharm
- Product numberBD157254
- Product descriptionZinc(II)sulfate 99%
- Packaging25g
- Price$19
- Updated2021-12-16
- Buy
- ManufacturerSigma-Aldrich
- Product number83265
- Product descriptionZinc sulfate solution BioUltra, for molecular biology, 2.0?M in H2O
- Packaging250ML
- Price$56.2
- Updated2024-03-01
- Buy
- ManufacturerSigma-Aldrich
- Product number1.09991
- Product descriptionZinc sulfate solution for 1000 ml, c(ZnSO?) = 0.1 mol/l (0.1 N) Titrisol?
- Packaging1amp
- Price$61.8
- Updated2024-03-01
- Buy
- ManufacturerSigma-Aldrich
- Product number1.08879
- Product descriptionZinc sulfate solution c(ZnSO4) = 0.1 mol/l, Titripur?, reag. Ph. Eur.
- Packaging1L
- Price$62.3
- Updated2024-03-01
- Buy
- ManufacturerSigma-Aldrich
- Product numberZ2876
- Product descriptionZinc sulfate solution 0.3N
- Packaging500ml
- Price$68.5
- Updated2024-03-01
- Buy
- ManufacturerSigma-Aldrich
- Product number83265
- Product descriptionZinc sulfate solution BioUltra, for molecular biology, 2.0?M in H2O
- Packaging1L
- Price$189
- Updated2024-03-01
- Buy
- ManufacturerTRC
- Product numberZ439985
- Product descriptionZinc sulfate
- Packaging250ml
- Price$120
- Updated2021-12-16
- Buy
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|
| Alfa Aesar | J60963 | Zinc sulfate 100mM aq. soln. | 100ml | $60.7 | 2023-06-20 | Buy |
| BLDpharm | BD157254 | Zinc(II)sulfate 99% | 5g | $7 | 2021-12-16 | Buy |
| BLDpharm | BD157254 | Zinc(II)sulfate 99% | 10g | $10 | 2021-12-16 | Buy |
| BLDpharm | BD157254 | Zinc(II)sulfate 99% | 25g | $19 | 2021-12-16 | Buy |
| Sigma-Aldrich | 83265 | Zinc sulfate solution BioUltra, for molecular biology, 2.0?M in H2O | 250ML | $56.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.09991 | Zinc sulfate solution for 1000 ml, c(ZnSO?) = 0.1 mol/l (0.1 N) Titrisol? | 1amp | $61.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.08879 | Zinc sulfate solution c(ZnSO4) = 0.1 mol/l, Titripur?, reag. Ph. Eur. | 1L | $62.3 | 2024-03-01 | Buy |
| Sigma-Aldrich | Z2876 | Zinc sulfate solution 0.3N | 500ml | $68.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | 83265 | Zinc sulfate solution BioUltra, for molecular biology, 2.0?M in H2O | 1L | $189 | 2024-03-01 | Buy |
| TRC | Z439985 | Zinc sulfate | 250ml | $120 | 2021-12-16 | Buy |
Properties
Melting point :100°C
Boiling point :105°C (estimate)
Density :1.31 g/mL at 20 °C
storage temp. :Store at +15°C to +25°C.
solubility :H2O: soluble
form :Liquid
color :Colorless
PH :4.0±0.5
Water Solubility :Soluble
λmax :λ: 260 nm Amax: <0.02
λ: 280 nm Amax: <0.02 Merck :14,10159
LogP :-1.031 (est)
CAS DataBase Reference :7733-02-0(CAS DataBase Reference)
NIST Chemistry Reference :Zinc sulfate(7733-02-0)
EPA Substance Registry System :Zinc sulfate (7733-02-0)
Boiling point :105°C (estimate)
Density :1.31 g/mL at 20 °C
storage temp. :Store at +15°C to +25°C.
solubility :H2O: soluble
form :Liquid
color :Colorless
PH :4.0±0.5
Water Solubility :Soluble
λmax :λ: 260 nm Amax: <0.02
λ: 280 nm Amax: <0.02 Merck :14,10159
LogP :-1.031 (est)
CAS DataBase Reference :7733-02-0(CAS DataBase Reference)
NIST Chemistry Reference :Zinc sulfate(7733-02-0)
EPA Substance Registry System :Zinc sulfate (7733-02-0)
Safety Information
| Symbol(GHS): |
  
|
||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal word: | Danger | ||||||||||||||||||||||||||||||||||||||||||
| Hazard statements: |
|
||||||||||||||||||||||||||||||||||||||||||
| Precautionary statements: |
|
Description
Zinc sulfate appears as colorless or white rhombic crystals or powder at room temperature. It has convergence property and is easily soluble in water with its aqueous solution being acidic. It is slightly soluble in ethanol and glycerol. Pure zinc sulfate can be stored in the air for a long time without turning yellow. It can lose water to become white powder when placed in dry air. There are various kinds of hydrates: in the range of 0-39 ° C, its stable hydrate balanced with aqueous phase is zinc sulfate heptahydrate; in the range of 39-60 ° C, it is hexahydrate zinc sulfate. At the range of 60-100 °C, it will become zinc sulfate monohydrate. When being heated to 280 °C, various kinds of hydrate will completely lose water with decomposition into zinc sulfate at 680 °C and further decomposition at above 750 ° C and finally decomposition into zinc oxide and sulfur trioxide at about 930 °C. ZnSO4 • 7H2O can form mixed crystal with MSO4 • 7H2O (M = Mg, Fe, Mn, Co, Ni) within a certain range. It is mainly used for the preparation of raw materials of pigment lithopone, zinc barium and other zinc compounds. It also has various kinds of applications such as animal nutrition upon zinc deficiency, animal feed additives, crop zinc fertilizer (trace element fertilizer), important materials of artificial fiber, electrolyte solution upon electrolytic production of zinc metal, mordant in the textile industry, pharmaceutical emetic agents, astringents, fungicides and wood and leather preservatives. It can be derived from the reaction between zinc or zinc oxide and sulfuric acid or from the baking of sphalerite in the baking furnace followed by extraction and refining.More related product prices
ZINC Potassium persulfate Neomycin sulfate SULFATE STANDARD Zinc sulfate monohydrate Atropine sulfate Copper(II) sulfate Ammonium sulfate Magnesium sulfate Zinc sulfate heptahydrate Colistin sulfate ZINC SULFIDE Ferric sulfate Zinc pyrithione ZINC Sulfuric acid Zinc sulphateRelated product price
- ZINC
$40-4352.57 - Potassium persulfate
$10-550 - Neomycin sulfate
$5-1651.1
Suppliers and manufacturers
Henan Tianfu Chemical Co.,Ltd.
Hubei Jusheng Technology Co.,Ltd.
Xiamen AmoyChem Co., Ltd
HubeiwidelychemicaltechnologyCo.,Ltd





