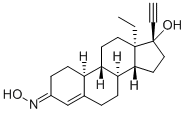
ノルエルゲストロミン
|
- ¥90100 - ¥283900
- 化学名: ノルエルゲストロミン
- 英語名: DEACETYLNORGESTIMATE (25 MG) ((E)- AND (Z)-17-DEACETYL NORGESTIMATE MIXTURE)
- 別名:[17R,(+)]-17-ヒドロキシ-13-エチル-18,19-ジノルプレグナ-4-エン-20-イン-3-オンオキシム;ノルエルゲストロミン;17-デアセチルノルゲスチマート;(17R)-13-エチル-17-ヒドロキシ-18,19-ジノル-プレグナ-4-エン-20-イン-3-オンオキシム;デアセチルノルゲスチメート ((E)- AND (Z)-17-DEACETYL NORGESTIMATE MIXTURE);(1R,3aS,3bR,9aR,9bS,11aS)-11a-エチル-1-エチニル-7-(ヒドロキシイミノ)-1H,2H,3H,3aH,3bH,4H,5H,7H,8H,9H,9aH,9bH,10H,11H,11aH-シクロペンタ[a]フェナントレン-1-オール
- CAS番号: 53016-31-2
- 分子式: C21H29NO2
- 分子量: 327.46
- EINECS:
- MDL Number:MFCD03695603
3物価
選択条件:
ブランド
- 富士フイルム和光純薬株式会社(wako)
- Sigma-Aldrich Japan
パッケージ
- 25mg
- 200mg
- 生産者富士フイルム和光純薬株式会社(wako)
- 製品番号W01USP1471958
- 製品説明デアセチルノルゲスチメート ((E)- and (Z)-17-deacetyl norgestimate mixture)
- 英語製品説明Deacetylnorgestimate ((E)- and (Z)-17-deacetyl norgestimate mixture)
- 包装単位25mg
- 価格¥283900
- 更新しました2023-06-01
- 購入
- 生産者Sigma-Aldrich Japan
- 製品番号1468454
- 製品説明 United States Pharmacopeia (USP) Reference Standard
- 英語製品説明Norelgestromin United States Pharmacopeia (USP) Reference Standard
- 包装単位200mg
- 価格¥90100
- 更新しました2024-03-01
- 購入
- 生産者Sigma-Aldrich Japan
- 製品番号1471958
- 製品説明 United States Pharmacopeia (USP) Reference Standard
- 英語製品説明Deacetylnorgestimate United States Pharmacopeia (USP) Reference Standard
- 包装単位25mg
- 価格¥170000
- 更新しました2023-06-01
- 購入
| 生産者 | 製品番号 | 製品説明 | 包装単位 | 価格 | 更新時間 | 購入 |
|---|---|---|---|---|---|---|
| 富士フイルム和光純薬株式会社(wako) | W01USP1471958 | デアセチルノルゲスチメート ((E)- and (Z)-17-deacetyl norgestimate mixture) Deacetylnorgestimate ((E)- and (Z)-17-deacetyl norgestimate mixture) |
25mg | ¥283900 | 2023-06-01 | 購入 |
| Sigma-Aldrich Japan | 1468454 | United States Pharmacopeia (USP) Reference Standard Norelgestromin United States Pharmacopeia (USP) Reference Standard |
200mg | ¥90100 | 2024-03-01 | 購入 |
| Sigma-Aldrich Japan | 1471958 | United States Pharmacopeia (USP) Reference Standard Deacetylnorgestimate United States Pharmacopeia (USP) Reference Standard |
25mg | ¥170000 | 2023-06-01 | 購入 |
プロパティ
融点 :107-109°C
沸点 :491.9±45.0 °C(Predicted)
比重(密度) :1.23±0.1 g/cm3(Predicted)
貯蔵温度 :Sealed in dry,2-8°C
溶解性 :DMF: 11 mg/ml; DMSO: 10 mg/ml; Ethanol: 12 mg/ml; PBS (pH 7.2): 0.25 mg/ml
外見 :A solid
酸解離定数(Pka) :12.22±0.60(Predicted)
CAS データベース :53016-31-2
沸点 :491.9±45.0 °C(Predicted)
比重(密度) :1.23±0.1 g/cm3(Predicted)
貯蔵温度 :Sealed in dry,2-8°C
溶解性 :DMF: 11 mg/ml; DMSO: 10 mg/ml; Ethanol: 12 mg/ml; PBS (pH 7.2): 0.25 mg/ml
外見 :A solid
酸解離定数(Pka) :12.22±0.60(Predicted)
CAS データベース :53016-31-2
安全情報
| 絵表示(GHS): |

|
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注意喚起語: | Danger | |||||||||||||||||||||
| 危険有害性情報: |
|
|||||||||||||||||||||
| 注意書き: |
|
説明
Ortho Evra@ is the first birth control transdermal patch and contains a combination of norelgestromin and ethinylestradiol. The patch can be applied to the buttocks, lower abdomen, upper torso or arms; changed weekly for 3 weeks, followed by a patch-free week. Norelgestromin is the active metabolite produced following oral administration of norgestimate, the progestin component of the contraceptive, Ortho-Cyclen?. The half-life value of norelgestromin was approximately 28 h. Following application, norelgestromin rapidly appeared in the serum, reached a plateau by approximately 48h and was maintained at an approximate steady state throughout the wear period. Hepatic metabolites of norelgestromin included norgestrel and various hydroxylated and conjugated metabolites that were eliminated by renal and fecal pathways. Ortho Evra produced ovarian suppression and cycle control. It was significantly more effective in suppressing follicular development than the leading oral contraceptive Triphasil? (ethinyl estradiol/levomorgestrel); was as effective in preventing pregnancies and had similar tolerability profile. The patch offers 99% efficacy when used appropriately, as shown by three clinical trials. However, a statistically significantly greater proportion of pregnancies occurred among women weighing 90 kg or more. Ortho Evra? was well tolerated, with 2.6% of women discontinuing treatment due to mild to moderate reactions at the site of application. Thus, the patch combined the efficacy of oral contraceptives with the convenience of just once-weekly dosing.関連商品価格
- 17-アセトキシプレグナ-4-エン-3,20-ジオン
¥4000-568800 - N-(ジクロロフルオロメチルチオ)-N-(ジメチルアミノスルホニル)アニリン
¥5300-201900 - 4-tert-ブチルフェニル(メチル)ケトン
¥4700-252500




