| Company Name: |
Hangzhou Bingochem Co., Ltd.
|
| Tel: |
0571-8512-5372 |
| Email: |
sales@bingochem.com |
| Products Intro: |
Product Name:0
CAS:20084-93-9
Purity:99% HPLC Package:1g; 5g; 25g; 1kg; 5kg; 25kg
|
|
| | slaframine Basic information |
| Product Name: | slaframine | | Synonyms: | slaframine;(1S,8aα)-6β-Aminooctahydroindolizin-1β-ol acetate;1-Indolizinol, 6-aminooctahydro-, 1-acetate, (1S,6S,8aS)-;(-)-Slaframine | | CAS: | 20084-93-9 | | MF: | C10H18N2O2 | | MW: | 198.26 | | EINECS: | | | Product Categories: | | | Mol File: | 20084-93-9.mol | 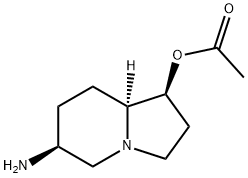 |
| | slaframine Chemical Properties |
| Boiling point | 278.5±35.0 °C(Predicted) | | density | 1.15±0.1 g/cm3(Predicted) | | pka | 10.11±0.40(Predicted) |
| | slaframine Usage And Synthesis |
| Description | Certain legume forages used for dairy cattle feeding stock have been known to produce excessive salivation when infested by Rhizoctonia legurninicola and this has been shown to be due to the presence of an indolizidine alkaloid, slaframine. The base is amorphous and also yields an amorphous dihydrochloride. The dipicrate, however, is crystalline, m.p. l83-4°C as is also the N-acetyl derivative, m.p. l43-6°C; [α]25D - 15.9° (c 5.0, EtOH). NMR and high-resolution mass spectrometry have shown that the earlier structure required revision to that given above, namely l-acetoxy-6-aminooctahydroindolizidine. The configuration has been demonstrated to be 1 S,6S,8aS-. | | Uses | (-)-Slaframine, is an indolizidine alkaloidal mycotoxin that generally causes salivation (slobbers) in most animals. It is usually produced by the fungus Rhizoctonia leguminicola. | | Definition | ChEBI: An indolizidine alkaloid that is octahydroindolizine substituted by an amino group at position 6 and an acetyloxy group at position 1. | | References | Rainey et al., Nature, 205, 203 (1965)
Aust, Broquist., ibid, 205, 204 (1965)
Aust, Broquist, Rinehart.,l. Arner. Chern. Soc., 88,2879 (1966)
Whitlock et al., Tetrahedron Lett., 3819 (1966)
Revised structure:
Gardiner et al., J. Arner. Chern. Soc., 90,5639 (1968) |
| | slaframine Preparation Products And Raw materials |
|