|
|
| | (5α,12β,19α,20R)-2,3-Didehydro-20-hydroxy-16-methoxyaspidospermidine-3-carboxylic acid methyl ester Basic information |
| Product Name: | (5α,12β,19α,20R)-2,3-Didehydro-20-hydroxy-16-methoxyaspidospermidine-3-carboxylic acid methyl ester | | Synonyms: | 16-Methoxyminovincinine;(5α,12β,19α,20R)-2,3-Didehydro-20-hydroxy-16-methoxyaspidospermidine-3-carboxylic acid methyl ester;Aspidospermidine-3-carboxylic acid, 2,3-didehydro-20-hydroxy-16-methoxy-, methyl ester, (5α,12R,19α,20R)- | | CAS: | 22341-28-2 | | MF: | C22H28N2O4 | | MW: | 384.48 | | EINECS: | | | Product Categories: | | | Mol File: | 22341-28-2.mol | 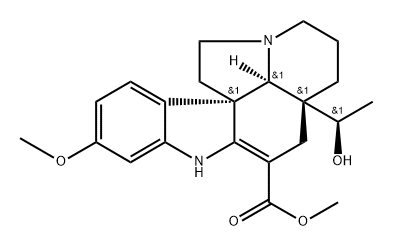 |
| | (5α,12β,19α,20R)-2,3-Didehydro-20-hydroxy-16-methoxyaspidospermidine-3-carboxylic acid methyl ester Chemical Properties |
| Boiling point | 519.1±50.0 °C(Predicted) | | density | 1.32±0.1 g/cm3(Predicted) | | pka | 14.88±0.20(Predicted) |
| | (5α,12β,19α,20R)-2,3-Didehydro-20-hydroxy-16-methoxyaspidospermidine-3-carboxylic acid methyl ester Usage And Synthesis |
| Description | A further alkaloid occurring in Vinca minor, this base is also amorphous and
laevorotatory having [0:]1>4 - 395° (c 0.2, CHCI3). The ultraviolet spectrum
contains two absorption maxima at 247 and 327 mfJ.. In addition to the methoxyl
and methoxycarbonyl groups, the alkaloid contains an imino group and a
secondary hydrozyl group. | | References | Dopke, Meisel, Spiteller., Tetrahedron Lett., 6065 (1968) |
| | (5α,12β,19α,20R)-2,3-Didehydro-20-hydroxy-16-methoxyaspidospermidine-3-carboxylic acid methyl ester Preparation Products And Raw materials |
|