- Dasabuvir
-

- $33.00 / 2mg
-
2025-07-29
- CAS:1132935-63-7
- Min. Order:
- Purity: 99.62%
- Supply Ability: 10g
- Dasabuvir
-

- $0.00 / 1g
-
2025-01-13
- CAS:1132935-63-7
- Min. Order: 1g
- Purity: More Than 99%
- Supply Ability: 100kg/Month
- Abt333
-

- $0.00 / 1g
-
2025-01-13
- CAS:1132935-63-7
- Min. Order: 1g
- Purity: More Than 99%
- Supply Ability: 100kg/Month
|
| | ABT-333 Basic information |
| Product Name: | ABT-333 | | Synonyms: | ABT-333;Dasabuvir (ABT-333);N-[6-[3-tert-Butyl-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-2-methoxyphenyl]naphthalen-2-yl]methanesulfonamide Dasabuvir (ABT-333);Dasabuvir;ABT-333/Dasabuvir;N-{6-[5-(2,4-Dioxo-3,4-dihydro-1(2H)-pyrimidinyl)-2-methoxy-3-(2-methyl-2-propanyl)phenyl]-2-naphthyl}methanesulfonamide;CS-2000;ABT333; ABT 333 | | CAS: | 1132935-63-7 | | MF: | C26H27N3O5S | | MW: | 493.57 | | EINECS: | | | Product Categories: | | | Mol File: | 1132935-63-7.mol | 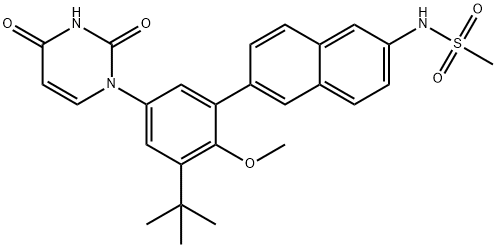 |
| | ABT-333 Chemical Properties |
| Melting point | >192oC (dec.) | | density | 1.317±0.06 g/cm3(Predicted) | | storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere | | solubility | Chloroform (Slightly, Heated), DMSO (Slightly), Methanol (Slightly, Heated) | | form | Solid | | pka | 8.52±0.30(Predicted) | | color | Pale Brown to Light Brown | | InChI | InChI=1S/C26H27N3O5S/c1-26(2,3)22-15-20(29-11-10-23(30)27-25(29)31)14-21(24(22)34-4)18-7-6-17-13-19(28-35(5,32)33)9-8-16(17)12-18/h6-15,28H,1-5H3,(H,27,30,31) | | InChIKey | NBRBXGKOEOGLOI-UHFFFAOYSA-N | | SMILES | CS(NC1=CC=C2C(=C1)C=CC(C1=CC(N3C(=O)NC(=O)C=C3)=CC(C(C)(C)C)=C1OC)=C2)(=O)=O |
| | ABT-333 Usage And Synthesis |
| Uses | Dasabuvir is an antiviral drug used in the treatment of chronic hepatitis C virus (HCV) infection. | | Definition | ChEBI: A member of the class of pyrimidone, which is (as the monohydrate of its sodium salt) in combination with ombitasvir, paritaprevir and ritonavir (under the trade name Viekira Pak) for treatment of chronic hepatitis C virus genotype 1 infection as well as c
rrhosis of the liver. | | Biological Activity | Dasabuvir is a nonnucleoside inhibitor of the RNA-dependent RNA polymerase encoded by the HCV NS5B gene, inhibits recombinant NS5B polymerases derived from HCV genotype 1a and 1b clinical isolates | | Clinical Use | Treatment of chronic hepatitis C infection | | Synthesis | S3: Under nitrogen protection, 1,4-dioxane (141.4 L), palladium catalyst Pd(PPh3)4 (3.9 kg) and water (70.68 L) were added to the reaction vessel, followed by N-(6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)naphthalen-2-yl)methanesulfonamide (23.56 kg) and 1-(3 -(tert-butyl)-5-iodo-4-methoxyphenyl)pyrimidine-2,4(1H,3H)-dione (27.13 kg) and mixed with stirring. Then sodium carbonate (8.63 kg) was added and the reaction solution was kept for 4-6 hours, during which the progress of the reaction was monitored by TLC until the reaction was completed. After completion of the reaction, the reaction solution was cooled to 40 °C and then diluted with ethyl acetate (235.6 L). The combined organic phases were washed with water (47.12 L), followed by concentration of the organic phase by distillation under reduced pressure at 50 °C. Ethyl acetate (94.24 L) was added to the crude product and stirred at 50-60 °C for 30 min to give a final crude product (29.45 kg). The crude product was pump-filtered and purified by silica gel column chromatography. The substitution reaction was a single step with 76.5% yield. | | Drug interactions | Potentially hazardous interactions with other drugs
Antibacterials: avoid concomitant use with
clarithromycin and telithromycin; concentration
possibly reduced by rifampicin - avoid.
Antidepressants: concentration possibly reduced by
St John’s wort - avoid.
Antiepileptics: concentration reduced by
carbamazepine - avoid; concentration possibly
reduced by fosphenytoin, phenobarbital, phenytoin
and primidone - avoid.
Antifungals: concentration of both drugs increased
with ketoconazole and possibly itraconazole and
posaconazole - avoid.
Diuretics: concentration of furosemide increased
(reduce furosemide dose).
Immunosuppressants: increases concentration of
ciclosporin (reduce ciclosporin dose by a fifth);
everolimus (avoid); sirolimus and tacrolimus (reduce
dose and use only if benefit outweighs risk - see
SPC).
Lipid-regulating drugs: avoid with atorvastatin,
gemfibrozil and simvastatin; concentration of
rosuvastatin increased (reduce dose of rosuvastatin).
Oestrogens: avoid concomitant use with
ethinylestradiol. | | Metabolism | Dasabuvir is mainly metabolised by CYP2C8 and to
a lesser extent by CYP3A. Seven metabolites were
identified in plasma. The most abundant plasma
metabolite was M1, which represented 21% of drug�related radioactivity (AUC) in circulation following single
dose; it is formed via oxidative metabolism predominantly
by CYP2C8.
Following a 400 mg 14C-dasabuvir dose, approximately
94% of the radioactivity was recovered in faeces with
limited radioactivity (approximately 2%) in urine.
Unchanged dasabuvir accounted for 26.2% and M1 for
31.5% of the total dose in faeces. M1 is mainly cleared
through direct biliary excretion with the contribution of
UGT-mediated glucuronidation and, to a small extent,
oxidative metabolism. | | References | [1] Patent: CN107266373, 2017, A. Location in patent: Paragraph 0057; 0058; 0059
[2] Patent: WO2009/39134, 2009, A1. Location in patent: Page/Page column 109
[3] Patent: WO2009/39127, 2009, A1. Location in patent: Page/Page column 168-169
[4] Patent: WO2009/39134, 2009, A1. Location in patent: Page/Page column 102-103; 109 |
| | ABT-333 Preparation Products And Raw materials |
|