|
|
| | Cenicriviroc Basic information |
| Product Name: | Cenicriviroc | | Synonyms: | Cenicriviroc;TAK652;Cenicriviroc mesylate
(TAK-652);Cenicriviroc(TAK652);TAK-652; TAK652; TAK 652; TBR-652; TBR 652; TBR652; CENICRIVIROC.;CS-2572;TAK-652; TBR-652; TAK652; TBR652; TAK 652; TBR 652;TBR 652 | | CAS: | 497223-25-3 | | MF: | C41H52N4O4S | | MW: | 696.94 | | EINECS: | | | Product Categories: | | | Mol File: | 497223-25-3.mol | 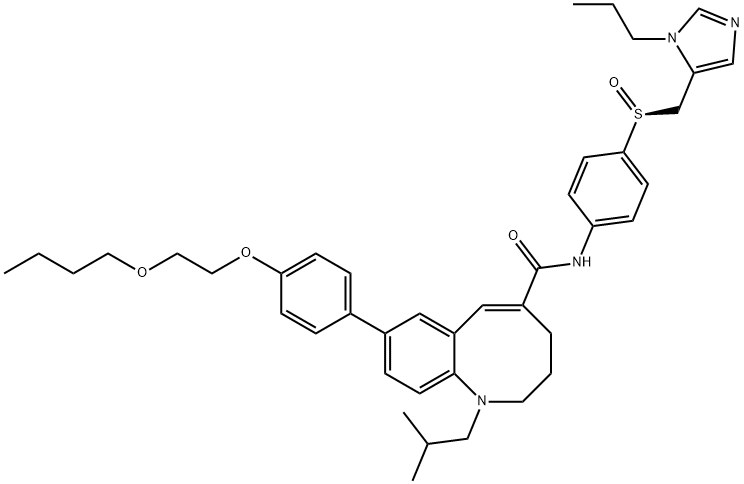 |
| | Cenicriviroc Chemical Properties |
| Boiling point | 913.5±65.0 °C(Predicted) | | density | 1.17±0.1 g/cm3(Predicted) | | storage temp. | Store at -20°C | | solubility | DMF: 20 mg/ml; DMSO: 20 mg/ml; DMSO:PBS (pH 7.2) (1:1): 0.5 mg/ml; Ethanol: 5 mg/ml | | form | A crystalline solid | | pka | 12.96±0.70(Predicted) |
| | Cenicriviroc Usage And Synthesis |
| Description | Cenicriviroc is a dual antagonist of chemokine receptor types 2 and 5 (CCR2/CCR5). The receptors are found on Kupffer cells and hepatic stellate cells, and preclinical studies suggest its anti-inflammatory and anti-fibrotic activity. In the phase 2b CENTAUR study, cenicriviroc treatment for 1 year failed to achieve the primary outcome of a decrease in NAFLD activity score by ≥2 points with no worsening of fibrosis. Nonetheless, one of the key secondary outcomes, improvement in liver fibrosis without worsening of NASH, was achieved in 20% in the treatment group, compared with 10% in the placebo group. Besides, cenicriviroc reduced the serum levels interleukin- 6, C-reactive protein and fibrinogen, suggesting its action on inflammation that may not be reflected by crude histological assessment. A phase 3 study is planned. | | Uses | Cenicriviroc is an experimental drug candidate for the treatment of HIV infection. It is an inhibitor of CCR2 and CCR5 receptors, allowing it to function as an entry inhibitor which prevents the virus from entering into a human cell. | | Definition | ChEBI: Cenicriviroc is a member of the class of benzazocines that is (5Z)-1,2,3,4-tetrahydro-1-benzazocine which is substituted by a 2-methylpropyl, N-{4-[(S)-(1-propyl-1H-imidazol-5-yl)methanesulfinyl]phenyl}carboxamide and 4-(2-butoxyethoxy)phenyl groups at positions 1, 5 and 8, respectively. It is a potent chemokine 2 and 5 receptor antagonist currently in development for the treatment of liver fibrosis in adults with nonalcoholic steatohepatitis (NASH). It has a role as a chemokine receptor 5 antagonist, an anti-HIV agent, a chemokine receptor 2 antagonist, an antirheumatic drug and an anti-inflammatory agent. It is a diether, a member of imidazoles, a sulfoxide, an aromatic ether, a secondary carboxamide and a benzazocine. | | Clinical Use | Cenicriviroc is a small molecule inhibitor of both CCR2 and CCR5, and has demonstrated safety in early trials of human patients afflicted with HIV-1, as well as a phase IIb trial (ClinicalTrials.gov NCT01338883). Cenicriviroc has also demonstrated therapeutic potential in animal models of liver fibrosis as well as renal fibrosis. A phase II trial of cenicriviroc in patients with NASH and liver fibrosis is planned to complete in late 2017 and (ClinicalTrials.gov NCT02217475). This trial, also called “CENTAUR: Efficacy and Safety Study of Cenicriviroc for the Treatment of Nonalcoholic Steatohepatitis (NASH) in Adult Subjects With Liver Fibrosis,” is a double-blind, placebo-controlled, randomized multinational study with the primary endpoint being histologic improvement of NASH activity score without worsening of fibrosis in patients with NASH and liver fibrosis. Results of this study are pending. An open label rollover extension study of participants in CENTAUR began in early 2017 and is ongoing (ClinicalTrials. gov NCT03059446).
There is evidence that CCRs are important in fibrosis in the liver, lung and kidney. At least one molecule that inhibits CCRs has demonstrable safety in human subjects. As such, blockade of CCRs with molecules such as Cenicriviroc represent another potential therapeutic avenue for the treatment of fibrotic inflammatory bowel disease. |
| | Cenicriviroc Preparation Products And Raw materials |
|