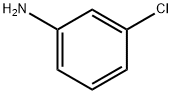
3-Chloroaniline
Nov 9,2021
General description
3-chloroaniline is a colorless to light amber liquid with a sweet odor. m.p.-11~-9 ℃, b.p.230~231 ℃ (95~96 ℃/1.47 kPa), n20D 1.5940, relative density 1.206, f.p.123 ℃. Insoluble in water, soluble in ethanol, ether, acid solution and organic solvents. M-Chloroaniline can be used as an intermediate of dyes, pesticides and medicines. Its hydrochloride is the base of ice dye dyes. It is mainly used as a developer for dyeing and printing cotton, linen and viscose fabrics. It can be used in medicine. Antipsychotic drugs chlorpromazine hydrochloride, perphenazine and chloroquine phosphate, etc. Meta-chloroaniline can also be used to make insecticides. Meta-chloroaniline is also an intermediate of the herbicide chloraniline, an intermediate of the medical diuretic and antihypertensive drug hydrochlorothiazide, and can be used to produce azo dyes. Therefore, metachloroaniline plays an irreplaceable role in organic synthesis.Hloroanilines are extensively used as the intermediates to produce rubber, dyes, plastic, cosmetics, pesticides, herbicides, preservatives and pharmaceuticals. The wide appli-cation of chloroanilines inevitably lead to them into different industrial wastewater, domestic sewage and agricultural runoff. Due to their high toxicity, large diffusion and low biodegradability, chloroanilines have been listed as important persistent toxic organic pollutants in many countries. The presence of chloroanilines could produce obvious toxicity to organisms, such as Phaeodactylum tricornutum, Oreochromis niloticus, Photobacteria Phosphoreum and Comamonas testosterone.3-Chlorotoluene is an organic intermediate, which can be used to synthesize pharmaceutical intermediates such as m-chlorobenzaldehyde, m-chlorobenzoic acid and m-chlorobenzyl chloride. At present, the main domestic production method is to diazotize diazonium hydrochloride through m-toluidine to generate diazonium hydrochloride, and then generate m-chlorotoluene through Sandmeier reaction.
.
Figure 1 the molecular formula of 3-Chloroaniline
Application and pharmacokinetics
Aromatic compounds are widely used in chemical and pharmaceutical industrie. These organic compounds contain chemically stable benzene ring structures and hence, their degradation by naturally occurring microbial communities in the contaminated environments is often slow. This is because the indigenous microbial community usually lacks efficient catabolic capabilities for these anthropogenic compounds. To efficiently remove these toxic compounds from contaminated water or soil, bioaugmentation has been a frequently investigated bioremediation approach, in which active bacteria capable of degrading recalcitrant organic compounds are introduced into the bioremediation system.
1.Bioaugmentation has been frequently proposed in wastewater and soil treatment to remove toxic aromatic compounds. The performance of bioaugmentation is affected by a number of biological and environmental factors including the interaction between the target pollutant and the augmented bacterial cells. In this study, using Comamonas testosteroni and 3-chloroaniline as the model organism and target pollutant, we explored the influence of toxic aromatic pollutant on the biofilm lifestyle of bacteria capable of degrading aromatic compounds toward a better understanding of cell-pollutant interaction in bioaugmentation. [1]
2.Chloroanilines from industrial wastewater can produce adverse effects on biological wastewater treatment systems due to their potential biotoxicity. The performance, nitrogen removal rate, microbial community and enzymatic activity of a sequencing batch reactor (SBR) were evaluated under transient 3-chloroaniline shock loading. The appearance of 3-chloroaniline promoted the microbial reactive oxygen species production and lactate dehydrogenase release. The transient 3-chloroaniline shock loading distinctly impacted the microbial richness and diversity. The present research results can provide theoretical basis and technical support for evaluating the effects of transient 3-chloroaniline shock on biological wastewater treatment systems, which is beneficial to take reasonable preventable measures to decrease the adverse effects on the bioreactor performance. [2]
Synthesis
The synthetic routes of m-chloroaniline include: m-chloronitrobenzene iron powder reduction method, m-chloronitrobenzene zinc powder reduction method, m-chloronitrobenzene sodium sulfide reduction method and Dichlorobenzene is produced by the ammonia decomposition of ammonia at low temperature. The m-chloronitrobenzene iron powder reduction method and the zinc powder reduction method produce a large amount of sludge; the process of ammonolysis of o-dichlorobenzene with ammonia at low temperature is complicated. The article refers to the sodium sulfide reduction method, using the 20% sodium hydrosulfide solution and part of the 70% sodium hydrosulfide prepared by the exhaust gas absorption by-products of the company’s production of thiocarbazide to prepare m-chloroaniline, even if the tail gas by-products are obtained. The treatment avoids the harmful effects of exhaust gas on the environment and has certain economic benefits.[3,4]
Figure 2 The Synthetic Route of M-chloroaniline
Toxicological and Safety
1. Skin/eye irritation Standard Draize test: rabbit, skin contact: 500mg/24H, severity of reaction: mild.Standard Draize test: rabbit, eye contact: 20mg/24H, severity of reaction: moderate.
2. Acute toxicity: rat oral LD50: 450mg/kg; mouse oral LD50: 740mg/kg; mouse oral LD50: 116mg/kg; rabbit oral LD50: 750mg/kg
3. Other multi-dose toxicity: rat oral TDLo: 8400mg/kg/30D-I;
4. The toxicological effects are similar to o-toluidine. Rat transplantation LD50 is about 150mg/kg. TJ 36-79 stipulates that the maximum allowable concentration in the air of the workshop is 5mg/m3.
5. Acute toxicity LD50: 450mg/kg (rat oral); 3250mg/kg (rabbit skin)
6. Irritation from fire.
Storage and Safty
M-Chloroaniline is a colorless to light yellow liquid, and the color becomes darker with light or long-term storage. This substance may be harmful to the environment, it is recommended not to let it enter the environment.
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The packaging is required to be sealed and not in contact with air. It should be stored separately from oxidants, acids, and edible chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with leakage emergency treatment equipment and suitable storage materials.
- Related articles
- Related Qustion
- 3-Chloroaniline: Applications in the Chemical Industry, Production Methods and Toxic Properties Feb 28, 2024
3-Chloroaniline, a key chemical intermediate, is vital for various industries despite its toxicity. Strict safety measures are crucial for handling to prevent adverse health effects.
- 3-Chloroaniline: applications and degradation pathways Oct 10, 2023
3-Chloroaniline is a chemical compound with detrimental effects on wastewater treatment systems, but can be degraded by bacteria, sunlight, or advanced oxidation processes for environmental safety.
(Trifluoromethyl)trimethylsilane belong to organic fluorine chemistry has made rapid progress in recent decades, and the research and development of fluorine-containing compounds have had a great impa....
Nov 9,2021Organic Synthesis IntermediateAmmonia is a naturally occurring compound which is a key component of the global nitrogen cycle and all living organisms. It is generated in nature primarily by the decay of plant and animal matter and is used as an essential nutrient by al....
Nov 9,2021Inorganic chemistry3-Chloroaniline
108-42-9You may like
- 3-Chloroaniline
-

- $30.00/ KG
- 2023-10-08
- CAS:108-42-9
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20T
- 3-Chloroaniline
-

- $10.00 / 1KG
- 2023-07-19
- CAS:108-42-9
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20 Tons
- 3-Chloroaniline
-

- $0.00 / 25KG
- 2023-06-20
- CAS:108-42-9
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month