3-isopropylphenol: Application, Chemicaland And Toxicological studies
Dec 26,2022
General description
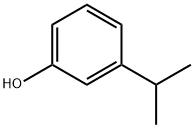
The 3-isopropylphenol, with the CAS No: 618-45-1,is also known as3-isopropylbenzenesulfonyl chloride. It belongs to the phenolic compound.This chemical’s molecular formula is C9H12Oand molecular weight is 136.19098,and as a ingredient of disinfectants.[1]


Figure 1 the molecular formula of3-isopropylphenol
Application
3-Isopropylphenyl N-methylcarbamate was prepd. by reaction of 3-isopropylphenol with methyl isocyanate.[2]The attraction of female tabanids to unbaited and single-baited canopy traps using 3-isopropylphenol was studied in three forest localities in eastern Croatia. Tabanids were collected in a significantly higher number in traps baited with these chemicals compared to unbaited control traps, and the results with 3-isopropylphenol is not significant for some tabanids. But, the 3-isopropylphenol baited traps collected 3.5times as many tabanids, than unbaited traps. [3]
During a comparative study of some cholinesterase inhibitors on the extra-cellularly recorded endplate potential of the rat phrenic diaphragm preparation it was observed that 3-isopropylphenylmethyIcarbamate (OMS 15) caused a slower rate of rise of the endplate potential than paraoxon, eserine, 2-methyl-2-methylthiopropion aldehydeo-(methylcarbamoy1)oxime (OMS 771) or 2-oxo-1,3-dithiolane @(methylcarbamoyl) oxime (OMS 744) when tested in concentrations which gave a similar degree of cholinesterase inhibition. It was thought likely that this difference could be due to a relative reduction of acetylcholine output from the phrenic nerve terminals in the presence of the phenylcarbamates.[4]Nonstaining, nonblooming, nonresinous antioxidants for rubber are made by condensation of 1 mole aliphatic aldehyde with 2 moles of C6H3(iso-Pr)(OH)(CRR'R''), where R is H or alkyl and R' and R'' are alkyl groups. Compds. prepd. were: the intermediates 2-tert-butyl-5-isopropylphenol , m. 49-50°, in 51-g. yield by addn. of Me3CCl to the mixt. of 68 g.
3-isopropylphenol and 4 g. SnCl4 in 50 ml. hexane at 40-50° during 2.5 hrs. and refluxing for 2 hrs.[5] Studies with eight 14C-carbonyl-labeled aryl methylcarbamates demonstrate that such insecticide chemicals are degraded with the carbamate moiety intact when applied to glass or silica gel surfaces or leaves of growing bean plants, or injected into the stems of such plants. Methylcarbamates-carbonyl-14C of the 3-isopropylphenol(UC 10854) were examd. Rates of loss vary considerably with the nature of the surface (inert or plant), the light, and the compd. Oxidative changes occur on and(or) in the plant: Mesurol degrades to sulfoxide and sulfone analogs; The fate of the radiocarbon, 6 days after injection into bean plants, varies considerably. With UC 10854, the majority of the radiocarbon is in the water phase; Loss from the plant surface is not directly related to the volatility of the compds., nor is degradation in the plant related to the rate of nonenzymic hydrolysis. It appears that the relative stability of the methylcarbamate grouping to photooxidn. and metabolism, in certain cases, allows the formation of degradation products involving only alteration of the ring or a ring substituent.[6]
Preparation
3-isopropylphenol, as a ingredient of disinfectants. 752 parts phenol and 15 parts fullers' earth, under N, were heated to 140℃and propylene gas was added at 4 L/min for 4.25h. The mixt. was cooled under N and the catalyst was filtered to give a liq. contg. Among, 3-isopropylphenol 0.63%.[1]When a single Me substituent at C-2 or C-4 of an o-quinol acetate is replaced by a halogen atom, a greatly decreased yield of the corresponding 3-isopropylphenol is obtained from reaction of the quinol acetate, e.g, with isopropylmagnesium bromide. Replacement of a second Me group by halogen results in a marked increase in the yield of the 3-isopropylphenol. Essentially identical product distributions are obtained from reactions of halogenated o-quinol acetates with concd. and dil. Grignard solns.[7]
Toxicity
Ests. of fish LC50 based on Microtox EC50 and fish LC50 relationships presented in the literature suggest that 4-isopropylphenol, 3-isopropylphenol, 2,4-diisopropylphenol, 2,5-diisopropylphenol, and thiocresol could threaten fish if exposure was equiv. to the low mg/L level in water. Fish LC50 would be expected to range from 0.01 to 0.4 mM based on extrapolation of EC50 for other substituted phenols.[8]A mixt. of 10 ppb thiophenol, 1 ppb 3-isopropylphenol, 1 ppb 2,4-diisopropylphenol, and 1 ppb carvacrol had nearly the same flavor quality as environmentally tainted northern pike (Esox lucius) when added to the flesh of untainted northern pike. Trout exposed to a mixt. of alkylphenols and thiophenol (1-2 ppb each) in water did not become flavor tainted, but trout fed a formulated feed (2% body wt./day) contg. 100 ppb added alkylphenols and thiophenol became strongly flavor tainted. Apparently, tainting via the food chain is important with these compds. Tainting caused by thiophenol and isopropylphenols in tank-held trout was removed by holding the fish in clean water for 5 days.[9]
References
[1] Cook A M. Phenolicdisinfectants[J]. Journal of Pharmacy & Pharmacology, 2011, 12(S1):19T-28T.
[2] Kilsheimer, J R, Hayes, H L. 3-Isopropylphenyl N-methylcarbamate insecticides[J]. 1960,GB85292019601102.
[3]Stjepan Krcmar. Responses of Tabanidae (Diptera) to canopy traps baited with 4-methylphenol,3-isopropylphenol, and naphthalene[J]. Comparative Study.2007, 32 (2): 188-92.
[4]Forshaw P J . Reduction of acetylcholine output from the indirectly stimulated rat diaphragm preparation by some carbamates and phenols[J]. Journal of Pharmacy and Pharmacology, 2011, 24(7):583-584.
[5]Aldehyde condensation products of mono-hydrocarbon-substituted m-isopropylphenol.(1957),GB76701919570130.
[6]Abdel-Wahab, A. M.; Kuhr, R. J.; Casida, J. E. Fate of 14C-carbonyl-labeled aryl methylcarbamate insecticide chemicals in and on bean plants[J]. Journal of Agricultural and Food Chemistry.1966,14(3),290-298.
[7]Miller B, Haggerty J G. Effects of halogen substitution on reactions of o-quinol acetates with isopropylmagnesium bromide and diisopropylmagnesium. Competition between unimolecular decomposition and bimolecular reactions of radical anions[J]. Journal of Organic Chemistry, 2002, 51(2):174-179.
[8]Heil T , Lane N , Lindsay R . Toxicological properties of thio- and alkylphenols causing flavor tainting in fish from the upper Wisconsin River[J]. Journal of Environmental Science & Health Part B, 1989, 24(4):361-388.
[9]Timothy, P, Heil, et al. Sensory properties of thio‐ and alkyl‐ phenols causing flavor tainting in fish from the upper Wisconsin River[J]. Journal of Environmental Science and Health, Part B, 1989.
- Related articles
- Related Qustion
- 3-Isopropylphenol: Harnessing Chemical Potentials While Navigating Toxicological and Safety Challenges Apr 15, 2024
3-Isopropylphenol, a compound known for its significant utility in the chemical synthesis and pharmaceutical industries.
Cetuximab is a human IgG1 monoclonal antibody that inhibits epidermal growth factor receptor , with a Kd of 0.201 nM for EGFR by SPR and has potent antitumor activity.....
Dec 26,2022APIFasudil hydrochloride is a potent Rho-kinase inhibitor and vasodilator. Since it was discovered, it has been used for the treatment of cerebral vasospasm, which is often due to subarachnoid hemorrhage, as well as to improve the cognitive....
Dec 26,2022API3-ISOPROPYLPHENOL
618-45-1You may like
3-ISOPROPYLPHENOL manufacturers
- 3-isopropylphenol
-

- $10.00 / 1Kg/Bag
- 2021-10-21
- CAS:618-45-1
- Min. Order: 1Kg/Bag
- Purity: 99%
- Supply Ability: 20 Tons
- 3-Isopropylphenol
-

- $10.00 / 1KG
- 2021-10-11
- CAS:618-45-1
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20 tons
- 3-ISOPROPYLPHENOL
-

- $1.00 / 1ASSAYS
- 2020-01-09
- CAS:618-45-1
- Min. Order: 1ASSAYS
- Purity: 85.0-99.8%
- Supply Ability: 20tons