A widely used coupling agent: Diphenylphosphoryl azide
Dec 1,2023
Description
Diphenylphosphoryl azide (DPPA)—a well-known azide reagent used in peptide couplings, Curtius rearrangements, and Mitsunobu inversions—is often encountered in pharmaceutical process development because it enables the most direct route to a desired product[1].
Chemical property
DPPA is an organic compound with the chemical formula C12H10N3O3P. This compound is a slightly yellow liquid and slightly acetonitrile or chloroform. DPPA has been used as a convenient reagent for racemization-free peptide synthesis since it was synthesized by Shioiri et al. in 1972. It is non-explosive and stable enough to be stored for a long time without decomposition.
Uses
diphenylphosphoryl azide (DPPA) could be used as an azide donor to synthesize ureas and their derivatives, like acyl ureas and thiocarbamates. N-acyl benzotriazole with the azide donor DPPA furnishes the corresponding acyl azide via nucleophilic substitution reaction during the reaction. This acyl azide intermediate undergoes Curtius rearrangement to furnish the corresponding isocyanate intermediate by the subsequent elimination of molecular N2. Further, the isocyanate intermediate is trapped by various nucleophiles to give the targeted ureas, carbamates, and thiocarbamates[2]. In this route, DPPA was an excellent alternative to trimethylsilyl azide and sodium azide for the azide donor in Curtius degradation.
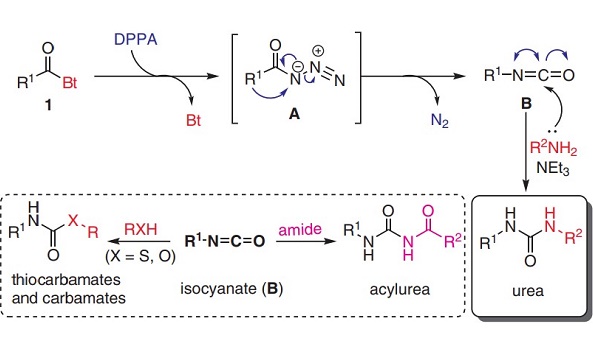
Figure 1. Plausible mechanism involving the Curtius rearrangement
This reagent can also be used successfully to polymerize amino acids or peptides. As a coupling agent, it is used to synthesize peptides in the presence of a base via the modified Curtis reaction. In 1991, Nishi et al. synthesized homopeptides using the DPPA method, but the composition was limited to one amino acid or one diamino acid[3].
A polypeptide can easily be prepared by stirring the solution or suspension of amino acid or peptide in a suitable solvent with DPPA and a tertiary amine such as triethylamine. Poly(amino acid)s such as poly(glycine), poly(L-alanine), poly(L-leucine), and poly(6-aminohexanoic acid) were prepared by this method. Nakajima et al. studied the polymerization reactions of L-alanylglycine with diphenylphosphoryl azide (DPPA) under various reaction conditions[4]. They found that dimethyl sulfoxide and triethylamine were the most suitable solvents and bases for this polymerization reaction. A reaction time of two days at room temperature, employing 1.3 equivalent moles of DPPA and 2.6 equivalent triethylamine, corresponding to the amount of L-alanyl glycine, was considered the most appropriate reaction condition.
References
[1] Stephen C. Born . “Continuous, on-demand generation and separation of diphenylphosphoryl azide.” Tetrahedron 74 25 (2018): Pages 3137-3142.
[2] V. Tiwari. “N-Acylbenzotriazoles as Proficient Substrates for an Easy Access to Ureas, Acylureas, Carbamates, and Thiocarbamates via Curtius Rearrangement Using Diphenylphosphoryl Azide (DPPA) as Azide Donor.” Synthesis-Stuttgart 46 1 (2021): 2494–2502.
[3] B. Nakajima, N. Nishi. “Polymerization of L-Alanylglycine with Diphenylphosphoryl Azide.” Polymer Journal 41 1 (1981): 183–189.
[4] Yuji Pu. “Using Diphenylphosphoryl Azide (DPPA) for the Facile Synthesis of Biodegradable Antiseptic Random Copolypeptides.” Macromolecular Rapid Communications 38 7 (2017).
- Related articles
- Related Qustion
- The performance of diphenylphosphoryl azide Jan 28, 2022
Diphenylphosphoryl azide, originally developed by Yamada in 1972, has shown significant synthetic versatility, being used in isocyanate synthesis.
- What is Diphenylphosphoryl azide? Apr 19, 2021
Diphenylphosphonic azide acts as a reagent for the synthesis of peptides and phosphoramidates by reacting with amines. It is also used in the preparation of oligosaccharides linked with carbamate and urea bonds.
Diphenyl ethers are used as carriers for commonly used dyes, as flavouring agents in food additives, as chemical intermediates in the heat transfer medium Dowtherm A and in surfactants and lubricants, et al.....
Dec 1,2023APIThe XeF4 Lewis structure consists of a central atom, xenon (Xe), and four outer atoms, fluorine (F), bonded at 90° and 180°. The xenon atom (Xe) and each fluorine atom (F) are connected by a single bond.....
Dec 1,2023APIDiphenylphosphoryl azide
26386-88-9You may like
- Benzhydrol:Melting point,Uses,Hazards
Mar 22, 2024
- What is Methoxypolyethylene glycol amine used for?
Mar 14, 2024
- What is 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine?
Mar 14, 2024
Diphenylphosphoryl azide manufacturers
- Diphenylphosphoryl azide
-

- $2.00 / 200KG
- 2024-04-26
- CAS:26386-88-9
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500mt/year
- Diphenylphosphoryl azide
-

- $30.00 / 1kg
- 2024-04-26
- CAS:26386-88-9
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 2000kg
- Diphenyl Azidophosphate (DPPA)
-

- $0.00 / 1Kg
- 2024-04-08
- CAS:26386-88-9
- Min. Order: 1Kg
- Purity: 99.9%
- Supply Ability: 200tons