Hydroxyprogesterone Caproate: History of Use, Pharmacokinetics and Safety
Apr 9,2024
General Description
Hydroxyprogesterone caproate, initially approved in 1956 for various conditions, gained prominence in preterm birth prevention in the 1960s. Despite early limitations, interest resurged in the 1990s, leading to pivotal trials like the Meis study confirming its efficacy. With a prolonged half-life and metabolism via CYP enzymes, its pharmacokinetic profile of Hydroxyprogesterone caproate varies among individuals. While crossing the placenta, its impact on clinical outcomes is still under investigation. Importantly, recent safety data from trials like PROLONG demonstrate its favorable safety profile, making Hydroxyprogesterone caproate a promising and safe option for preventing recurrent preterm birth without significantly increasing adverse event risks.

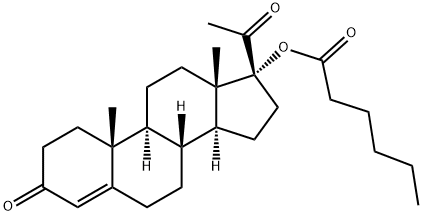
Figure 1. Hydroxyprogesterone caproate
History of use
Hydroxyprogesterone caproate has a rich history of use in the medical field. Initially approved by the FDA in 1956 under the trade name Delalutin for the treatment of menstrual disorders, uterine cancer, and miscarriage, its application in preterm birth prevention emerged in the 1960s. Early reports, including a study in Paris, France, demonstrated the potential of intramuscular progesterone in reducing the risk of preterm birth among high-risk women. However, the 1960s and 1980s saw limited clinical application due to inconsistent results, poor study design, and safety concerns. In 1990, a meta-analysis by Keirse et al reignited interest in progesterone use for preterm birth prevention, leading to a series of trials in the early 2000s. One significant trial conducted by the NICHD Maternal–Fetal Medicine Units Network, led by Meis et al, demonstrated a reduction in the rate of recurrent spontaneous preterm birth with Hydroxyprogesterone caproate injections. This pivotal trial, where 463 women with a history of spontaneous preterm birth were randomized, showcased a notable decrease in preterm birth rates. The positive outcomes of these trials have led to the current-day (2017) recommendations for the use of Hydroxyprogesterone caproate in clinical practice. As a result, hydroxyprogesterone caproate has evolved from its original indications to become a crucial prophylactic agent for preterm birth, offering hope and support to women at risk of premature delivery. 1
Pharmacokinetics
Hydroxyprogesterone caproate exhibits distinct pharmacokinetic characteristics that influence its efficacy and variability in clinical outcomes. This synthetic progestin, derived from 17-alpha hydroxyprogesterone with the addition of a caproate group, features a prolonged half-life compared to natural progesterone due to the caproate modification. Administered intramuscularly at a common dose of 250 mg every 7 days, Hydroxyprogesterone caproate is lipophilic, highly protein-bound, and stable under various conditions such as strong acid, high temperatures, and light exposure. Metabolized primarily by the cytochrome P450 (CYP) enzyme system, specifically CYP3A5, Hydroxyprogesterone caproate demonstrates significant inter-individual variability in metabolism and clearance rates. Peak concentrations are typically reached within 1-2 days post-administration, with a variable half-life ranging from 10 to 16 days. Factors such as maternal body mass index, race, and gestational status can impact the pharmacokinetics of Hydroxyprogesterone caproate, leading to differences in drug concentrations among individuals. Despite crossing the placenta and being detectable in cord blood plasma for an extended period, the relationship between Hydroxyprogesterone caproate levels and clinical outcomes remains a subject of ongoing research. Studies have shown that lower plasma concentrations of Hydroxyprogesterone caproate may be associated with a higher risk of recurrent preterm birth, emphasizing the importance of understanding individual drug levels for optimizing therapeutic efficacy. While efforts to establish a minimum effective concentration threshold for Hydroxyprogesterone caproate continue, routine monitoring and dose adjustments based on drug levels are not yet standard practice pending further research advancements in this area. 1
Safety
Hydroxyprogesterone caproate has shown promising safety outcomes in the prevention of recurrent preterm birth. Safety data from the Meis trial and the recent PROLONG trial indicate that Hydroxyprogesterone caproate does not significantly increase the risk of adverse events compared to a placebo. In the integrated dataset, the relative risks for miscarriage, stillbirth, neonatal deaths, and gestational diabetes were either lower or comparable to those of the placebo group. These findings highlight a favorable safety profile for Hydroxyprogesterone caproate, suggesting its use as a safe option for preventing recurrent preterm birth without substantially raising the risk of adverse events. 2
Reference
1. Manuck TA. 17-alpha hydroxyprogesterone caproate for preterm birth prevention: Where have we been, how did we get here, and where are we going? Semin Perinatol. 2017; 41(8): 461-467.
2. Sibai B, Saade GR, Das AF, Gudeman J. Safety review of hydroxyprogesterone caproate in women with a history of spontaneous preterm birth. J Perinatol. 2021; 41(4): 718-725.
- Related articles
- Related Qustion
- What is hydroxyprogesterone caproate? Sep 1, 2023
Hydroxyprogesterone caproate is a synthetic pregnancy agent and can be used to prevent or delay preterm birth in pregnant women
Polyquaternium-7 is a synthetic polymer that is widely used as a conditioning agent in the cosmetic industry.....
Apr 9,2024APIThymalfasin, chemically known as thymosin alpha 1, is a synthetic peptide that mirrors the biological activity of a naturally occurring thymic hormone.....
Apr 9,2024APIHydroxyprogesterone caproate
630-56-8You may like
Hydroxyprogesterone caproate manufacturers
- hydroxyprogesterone caproate
-

- $11.00 / 1kg
- 2024-04-30
- CAS:630-56-8
- Min. Order: 0.10000000149011612kg
- Purity: 99%
- Supply Ability: 10 tons
- hydroxyprogesterone caproate
-

- $10.00 / 1kg
- 2024-04-30
- CAS:630-56-8
- Min. Order: 10kg
- Purity: 98%
- Supply Ability: 20ton
- 17a-Hydroxyprogesterone caproate
-

- $0.00 / 1KG
- 2024-03-16
- CAS:
- Min. Order: 100g
- Purity: 98%+
- Supply Ability: 100kg