Mechanism and Side effects of Ciclopirox
Mar 31,2022
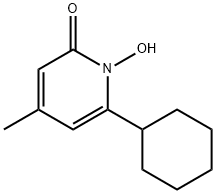
Ciclopirox is a synthetic hydroxypyridone, and ciclopirox olamine is the ethanolamine salt of ciclopirox. Its chemical name is the 2-aminoethanol salt of 6-cyclohexyl-1-hydroxy-4-methyl-2(1H)-pyridone; the chemical structure of ciclopirox is shown below. Ciclopirox has a broad spectrum of antifungal activity with a distinct mechanism of action. It is used to treat a variety of fungal infections including tinea pedis, tinea corporis/cruris, pityriasis versicolor, and seborrheic dermatitis and onychomycosis.
Mechanism of action
The mechanism of action of ciclopirox is diverse, targeting different metabolic processes in the fungal cell. Unlike azoles and allylamines, ciclopirox does not affect sterol synthesis. In contrast, it chelates trivalent cations (such as Fe3+), inhibits metal-dependent enzymes that are responsible for the degradation of toxic metabolites in the fungal cells, and targets diverse metabolic (e.g. respiratory) and energy-producing processes in microbial cells.
Moreover, ciclopirox inhibits cellular uptake of essential nutrients and alters cell membrane permeability at high concentration. Ciclopirox olamine has also been shown to significantly reduce the adherence of C. albicans to both buccal and vaginal epithelial cells at subinhibitory concentrations. Ciclopirox also displays a topical anti-inflammatory activity. This has been demonstrated in human polymorphonuclear cells in which ciclopirox was shown to inhibit prostaglandin and leukotriene synthesis.
Bioavailability
Approximately 1.3% of a dose of 1% ciclopirox olamine cream applied topically to the skin is absorbed into the systemic circulation, with peak serum concentrations of 0.01 mg/ml achieved 6 hours after application. Vaginal application of 5 g ciclopirox olamine cream for 1 week in women with vaginitis was associated with 15–20% absorption into the systemic circulation. In contrast, Coppi et al. (1993) found a low intravaginal absorption of ciclopirox olamine in women and an intravaginal bioavailability of 2% in rabbits. The drug is highly protein bound. It has a short half-life of 1.7 hours.
Side effects
Ciclopirox is generally safe and well tolerated. Most common sideeffects reported from clinical trials include burning sensation of the skin, irritation, redness, pain, and pruritus, which were transient and have rarely led to discontinuation of treatment. Two reports of allergic contact dermatitis secondary to ciclopirox topical application have been described in the literature. Treatment of the dermatitis and discontinuation of ciclopirox produced healing in both cases.
- Related articles
- Related Qustion
Amorolfine is available for topical use in 0.25% cream and 5% nail lacquer formulation for the treatment of onychomycosis in European countries and Japan. However, amorolfine is not approved in the USA for such indications. The major advant....
Mar 31,2022APIRilopirox, 6-[[p-(p-chlorophenoxy)phenoxy]methyl]-1-hydroxy-4- methyl-2(1H)-pyridone, is a new water insoluble fungicidal antimycotic. It is the second antifungal agent of the hydroxypyridone antifungals, along with ciclopirox. In contrast....
Mar 31,2022APICiclopirox
29342-05-0You may like
- Ciclopirox
-

- $0.00 / 1KG
- 2024-03-16
- CAS:29342-05-0
- Min. Order: 100g
- Purity: 98%+
- Supply Ability: 100kg
- Ciclopirox
-

- $0.00 / 25KG
- 2023-10-16
- CAS:29342-05-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month
- Ciclopirox
-

- $0.00 / 1KG
- 2023-09-06
- CAS:29342-05-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500000kg