Mechanism of action of Tipranavir
Apr 2,2022
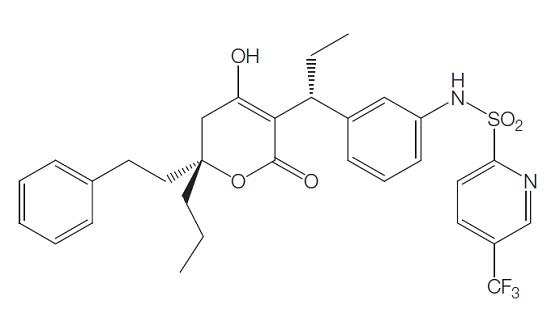
Tipranavir (formerly known as PNU-140690) is the first of a new class of nonpeptide protease inhibitors, the dihydropyrone sulfonamides. Tipranavir was developed and is marketed by Boehringer Ingelheim with the trade name Aptivuss. It was synthesized from coumarin and sulfonamide compounds as well as others. The chemical structure is shown below. The chemical name is 2-pyridinesulfonamide, N-[3-[(1R)-1-[(6R)-5, 6-dihydro-4-hydroxy-2-oxo-6-(2 phenylethyl)- 6-propyl-2H-pyran-3-yl]propyl]phenyl]-5-(trifluoromethyl). The chemical formula is C31H33F3N2O5S and the molecular weight is 602.7.
Uses
Ritonavir-boosted tipranavir is indicated for the treatment of HIV-1- infected adults (and children in the USA). It is recommended only for use in combination antiretroviral regimens for highly treatmentexperienced patients or those infected with HIV-1 strains resistant to multiple protease inhibitors and susceptible to tipranavir.
Mechanism of action
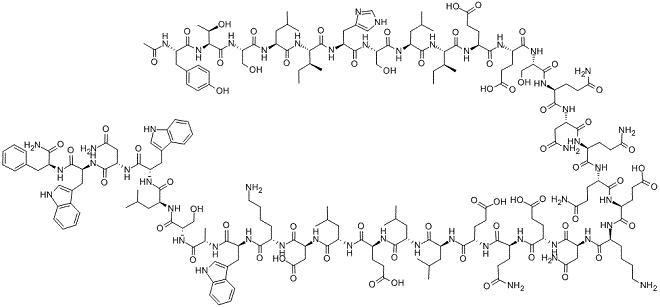
Like other HIV protease inhibitors, tipranavir binds to the active site of the HIV protease enzyme, thereby preventing processing of viral encoded Gag and Gag–Pol polyproteins necessary for the formation of mature, infectious viral particles. Tipranavir binding to the HIV-1 protease is highly selective. The tipranavir R-isomer inhibits recombinant HIV-1 protease with a Ki=8 pM, whereas against the HIV-2 protease it has a Kio2 nM, making it more than 100-fold less active against HIV-2 than against HIV-1. Muzammil et al. (2007) reported potent inhibition of wild-type HIV-1 protease with a Ki=19 pM. Ki values for tipranavir are in the mM range for human aspartic acid proteases (i.e. the human enzymes are a further 10-fold less susceptible than the HIV-2 protease).
X-ray crystallography of tipranavir in complex with the active site of HIV-1 protease shows that it forms a network of hydrophobic bonds with active site amino acids. Some of these interactions lead to the selection of active site mutations such as V82L/T and I84V, which are common resistance mutations shared by most of the protease inhibitors. Importantly, strong hydrogen bonds are also established with invariant regions of the enzyme including catalytic codon D25 and the backbone codons of D29, D30, G48, and I50 in the flap region. Binding to these residues is maintained in the face of mutant virus.
Thermodynamic studies suggest that tipranavir’s potency may be the result of its unique binding characteristics. High binding affinity is achieved when the changes in both enthalpy (thermodynamic potential or heat content) and entropy (amount of energy not available for doing work) contribute favorably to binding. Tipranavir exhibits a large favorable entropy change and a small but still favorable enthalpy change. As a result, tipranavir retains its inhibitory effects on the mutant HIV-1 protease with only an overall small loss in protease binding affinity. This is in contrast to most other protease inhibitors that exhibit a significant loss in binding affinity in the presence of mutant virus.
Bioavailability
Tipranavir is probably poorly absorbed in humans, like many protease inhibitors, although exact quantification of tipranavir absorption is unavailable. In a group of nine healthy male volunteers given radiolabeled tipranavir over a period of up to 20 days, the median total recovery of radioactivity was 87%, with 82% of the total radioactivity recovered in feces and less than 5% recovered from urine. Unchanged tipranavir accounted for 98.4–99.7% of plasma radioactivity.
In rats given radiolabeled tipranavir boosted with ritonavir, fecal excretion after intravenous tipranavir was about 85% and after oral administration almost 80%, whereas urinary excretion was 5% after intravenous administration and 9% after oral administration; these data confirm the presumed low oral bioavailability in humans.
Tipranavir is both a substrate for and a potent inducer of the intestinal P-glycoprotein (P-gp) efflux pump. Ritonavir inhibits P-gp. In vivo data suggest that co-administration of tipranavir with ritonavir at the 500/200 mg dose currently recommended leads to net induction of P-gp. Therefore, although ritonavir increases tipranavir bioavailability, net induction of P-gp by tipranavir may decrease absorption of other drugs that are substrates of P-gp, such as some of the other protease inhibitors.
Side effects
The most common side-effects seen in phase III trials with tipranavir– ritonavir were gastrointestinal, including diarrhea (23.2%) and nausea (16.5%). Other less frequent side-effects (ranging from 6.9% to 15.1%) were fever, fatigue, vomiting, and headache. The frequency of adverse events was similar for tipranavir and comparator protease inhibitors in the phase III trials conducted in antiretroviralexperienced patients. The total incidence of adverse events was 82.5% in the tipranavir arms vs 77.2% in the comparator arms. The number of patients who discontinued therapy due to adverse events was somewhat higher in the tipranavir arm, 12.4% vs 10.6% in the comparator protease inhibitor arm.
Hepatotoxicity
Tipranavir has a United States Food and Drug Administration"black
box" warning (reserved for serious adverse events) in relation to
reports of tipranavir-related hepatitis and hepatic decompensation,
some of which had a fatal outcome. Pooled data from phase III trials
show that patients receiving tipranavir–ritonavir had significantly
higher rates of grade 3 or 4 elevations of alanine aminotransferase
(ALT) and aspartate aminotransferase (AST), approximately 2- to
3-fold higher than seen in subjects taking other protease inhibitors.
- Related articles
- Related Qustion
Atazanavir (ATV) sulfate, (3S,8S,9S,12S)-3,12-bis(1,1-dimethylethyl)- 8-hydroxy-4,11-dioxo-9-(phenylmethyl)-6-[[4-(2-pyridinyl)phenyl] methyl]-2,5,6,10,13-pentaazatetradecanedioic acid dimethyl ester sulfate (1:1), previously known as BMS-2....
Apr 2,2022APIEnfuvirtide is an antiretroviral drug used in salvage regimens in (usually) highly drug-experienced individuals that suppresses replication of HIV-1 strains with multidrug resistance to reverse transcriptase inhibitors and protease inhibito....
Apr 2,2022APITipranavir
174484-41-4You may like
- Tipranavir
-

- $15.00 / 1KG
- 2021-07-13
- CAS:174484-41-4
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
- Tipranavir
-

- $15.00 / 1KG
- 2021-07-10
- CAS:174484-41-4
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
- Tipranavir
-

- $3.00 / 3KG
- 2019-07-12
- CAS:174484-41-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 100kg