Side effects of Enfuvirtide
Apr 2,2022
Enfuvirtide is an antiretroviral drug used in salvage regimens in (usually) highly drug-experienced individuals that suppresses replication of HIV-1 strains with multidrug resistance to reverse transcriptase inhibitors and protease inhibitors. It is used to optimize the response in these individuals to new HIV combination regimens.
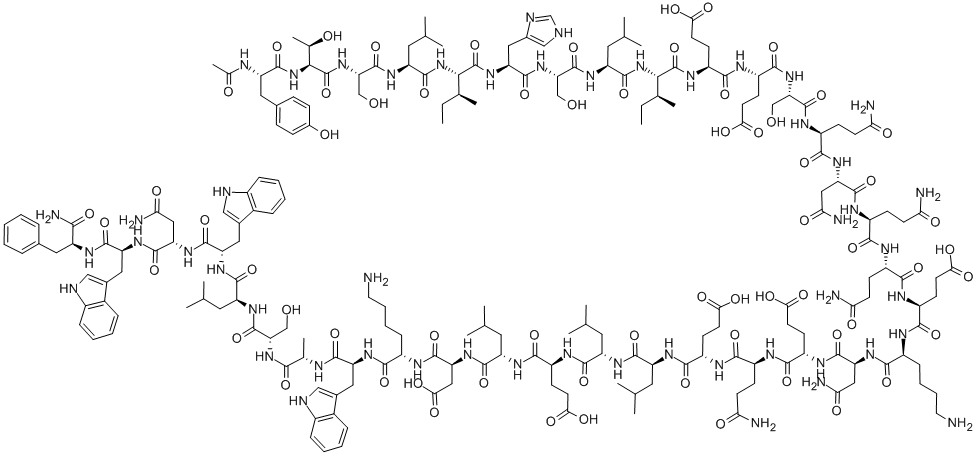
Enfuvirtide is a 36 amino acid peptide with a sequence analogous to the C-terminal heptad repeat (HR)-2 region of the viral surface glycoprotein gp41. Its mechanism of action is via competitive binding to the HR1 region of the gp41, preventing the interaction between HR1 and HR2 and impeding the conformational changes in the gp41 necessary for fusion of the virus with the cell. Therefore, the drug interferes with gp41 reorganization, thus blocking the fusion of viral and host cell membranes.
Uses
Enfuvirtide is a very useful and potent antiretroviral drug that is used almost exclusively as salvage therapy in HIV-1 infection. This is largely because of the requirement for parenteral administration. The factors associated with a good virologic response with salvage therapy are the availability of two or more active drugs in addition to enfuvirtide, previous antiretroviral experience with fewer than ten drugs, a baseline viral load of o100,000 copies/ml, and a basal CD4+ Tcell count of W100 cells/mm3. In TORO 1 and 2, RESIST, POWER, and BENCHMRK trials (all described below), an optimized antiretroviral regimen with more than one active drug plus enfuvirtide showed better virologic and immunologic responses than optimized regimen without enfuvirtide.
Mechanism of action
The process of HIV-1 entry into target cells is complex, involving multiple steps. It is initiated when the virus recognizes the CD4 receptor on the surface of the CD4+ T-cell by interacting initially with the viral glycoprotein gp120. The viral gp160 has two components: gp120, for the union with the CD4 receptor and HIV-1 chemokine coreceptors (CCR5 and CXCR4), and gp41, with two domains (HR1 and HR2), which undergo structural reorganization, forming a thermostable repeat six-helix structure which is critical for fusion of viral and cellular membranes. The mechanism of action of enfuvirtide is based on its binding to the HR1 in gp41, preventing it from interacting with the HR2 region of the virus, and thus inhibiting fusion of the viral and cell membrane and blocking the start of viral replication. The fusion step allows the injection of viral RNA into the target cell, which ultimately leads to the reverse transcription of viral RNA, migration of proviral DNA to the nucleus and integration into the host DNA.
Bioavailability
The estimated bioavailability of enfuvirtide is 84.3715.5% after subcutaneous administration when it is compared with intravenous administration of a 90-mg dose. The absorption rate from the selected subcutaneous sites is linear, and remains proportional to the administered dosage (in a dose range from 45 to 180 mg). No significant absorption difference has been demonstrated among the different areas recommended for subcutaneous administration. Enfuvirtide has high plasma protein binding (92%). Age does not affect enfuvirtide exposure in children and adolescents.
Side effects
During clinical trials, the majority of the patients treated with enfuvirtide developed local reactions at the site of injections. In the T-20 vs optimized regimen only (TORO) trials, 98% of patients receiving enfuvertide suffered erythema, nodules, induration, or local discomfort, but these reactions were treatment-limiting in not more than 4% of patients. The lesions may be transient and resolve within several weeks or stably persist beyond 30 days, with a chronic scleroderma-like pattern histologically. Using the needle-free injection system, painful injection site reactions are reduced by about 50% (35.7% vs 71.4%) and are less severe.
- Related articles
- Related Qustion
- Pharmacokinetics of Enfuvirtide Apr 29, 2022
Enfuvirtide (INN) is an HIV fusion inhibitor, the first of a class of antiretroviral drugs used in combination therapy for the treatment of HIV-1 infection. It is marketed under the trade name Fuzeon (Roche).
Tipranavir (formerly known as PNU-140690) is the first of a new class of nonpeptide protease inhibitors, the dihydropyrone sulfonamides. Tipranavir was developed and is marketed by Boehringer Ingelheim with the trade name Aptivuss. It was s....
Apr 2,2022APIMaraviroc was synthesized by Pfizer Inc as UK-427,857 (4, 4-difluoro- N-[(1S)-3-[exo-3-(3-isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo[ 3.2.1]oct-8-yl]-1-phenylpropyl]cyclohexanecarboxamide). The molecular weight of maraviroc is 5....
Apr 2,2022APIEnfuvirtide
159519-65-0You may like
- Enfuvirtide T-20
-

- $10.00 / 1kg
- 2024-03-08
- CAS:159519-65-0
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000kg
- Enfuvirtide
-

- $70.00 / 1kg
- 2024-01-02
- CAS:159519-65-0
- Min. Order: 10kg
- Purity: 0.99
- Supply Ability: 20tons
- enfuvirtide
-

- $40.00 / 1kg
- 2023-09-18
- CAS:159519-65-0
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 10 tons