Ninhydrin hydrate: history and applications
Nov 24,2023
General Description
Ninhydrin hydrate, synthesized in 1910 by Siegfried Ruhemann, has a fascinating history. Originally discovered accidentally, it was later found to react with ammonia and amines, leading to its popularity in detecting amino acids. In 1954, it was recognized as a reagent for developing fingerprints. Over the years, improvements have been made to enhance its application, including the development of ninhydrin analogues with better optical properties. It reacts with primary amino groups to form Ruhemann's purple, making it valuable in various scientific disciplines such as forensics, biochemistry, and nutrition. Ninhydrin hydrate reactions have been adapted for specialized needs and contribute to the advancement of analytical techniques.

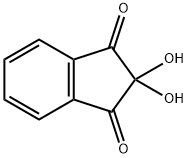
Figure 1. Ninhydrin hydrate
History
Ninhydrin hydrate, a crucial compound in chemistry, has an intriguing history dating back to its synthesis in 1910 by Siegfried Ruhemann, a distinguished professor of chemistry at Cambridge University. Originally, Ruhemann intended to create dicarbonyl compounds but unexpectedly isolated 1,2,3-indanetrione (ninhydrin) instead. He later found that this compound reacted with ammonia and amines to produce colored products. Moreover, Ruhemann's realization of Ninhydrin hydrate's structural similarities to alloxan and its ability to react with amino acids to yield a blue compound, murexide, led to the deduction that the product of the reaction between Ninhydrin hydrate and amino acids should have a similar structure. Following this discovery, Ninhydrin hydrate gained popularity for detecting and quantifying amino acids. However, it wasn't until 1954 that two Swedish scientists, Oden and von Hofsten, recognized its potential as a reagent for developing fingerprints. This historical progression highlights the accidental nature of scientific discovery and how unexpected findings can lead to significant advancements in diverse fields. 1
Applications
Reagent for developing latent fingerprints
Ninhydrin hydrate, originally used as a reagent for developing latent fingerprints, has undergone significant improvements in its application over the years. Forensic scientists initially sought a more sensitive reagent with reduced background staining and safer solvent mixtures. Despite formulation enhancements over four decades, the limitations of ninhydrin persisted, particularly in achieving improved contrast and visualization, especially on dark surfaces and coated paper. To address these shortcomings, new reagents were developed, such as the first three ninhydrin analogues reported by Almog et al in 1982. These analogues demonstrated that modifying the aromatic ring could enhance the optical properties of ninhydrin while maintaining its reactivity. By introducing various substituents and extending the conjugated system, these analogues exhibited increased molar extinction coefficients, resulting in more sensitive reagents with shifts in absorption maxima and variations in the color of the Ruhemann’s purple product. This diversity of ninhydrin-based reagents allows for optimal fingerprint development across different background colors and surfaces. Moreover, the development of more hydrophobic reagents facilitates easier formulation in less polar solvent systems. 2
Reaction with primary amino groups
Ninhydrin hydrate is a chemical compound that has found widespread applications in various scientific disciplines. It is known for its reaction with primary amino groups, forming a purple dye called Ruhemann's purple (RP). This reaction has been extensively studied and utilized in fields such as agricultural, biochemical, clinical, environmental, food, forensic, histochemical, microbiological, medical, nutritional, plant, and protein sciences. The unique characteristic of ninhydrin reactions is that they result in the formation of the same soluble chromophore by all primary amines at pH 5.5. This includes amines, amino acids, peptides, proteins, and even ammonia. Unlike other chromogenic reactions, the chromophore formed is not chemically bound to the protein or insoluble material, allowing it to be retained even after removing the insoluble substrate. Ninhydrin reactions have been adapted for specialized needs in analytical chemistry and biochemistry, including the use of acid, alkaline, and fluorogenic ninhydrin reagents. These adaptations have expanded the range of compounds that can be detected, isolated, and analyzed using ninhydrin. This chemical reaction has proven valuable in the detection and analysis of various compounds of interest. It provides a scientific basis for further improvements in analytical techniques and contributes to the cross-fertilization of information among different scientific disciplines. The historical perspective, chemistry and mechanisms, applications, and research needs related to ninhydrin reactions have been extensively studied and documented to enhance its utility in diverse scientific fields. 3
Reference
1. Hansen DB, Joullie MM. The development of novel ninhydrin analogues. Chem Soc Rev. 2005 May;34(5):408-417.
2. Almog J, Hirshfeld A, Klug JT. Reagents for the chemical development of latent fingerprints: synthesis and properties of some ninhydrin analogues. J Forensic Sci. 1982 Oct;27(4):912-917.
3. Friedman M. Applications of the ninhydrin reaction for analysis of amino acids, peptides, and proteins to agricultural and biomedical sciences. J Agric Food Chem. 2004 Feb 11;52(3):385-406.
- Related articles
- Related Qustion
Exposure to organic solvents such as NMP may result in neurological toxicity, inhalation in high concentrations causing headache, giddiness, mental confusion and nausea, and ingestion abdominal pain, nausea and vomiting.....
Nov 23,2023APIRucaparib, a potent PARP inhibitor, demonstrates strong anti-cancer activity but poses potential adverse effects.....
Nov 24,2023APINinhydrin hydrate
485-47-2You may like
Ninhydrin hydrate manufacturers
- Ninhydrin
-

- $0.00 / 1kg
- 2023-11-06
- CAS:485-47-2
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 50000kg
- Ninhydrin hydrate
-

- $9.00 / 1KG
- 2023-09-12
- CAS:485-47-2
- Min. Order: 1KG
- Purity: 99.9
- Supply Ability: 1 ton
- Ninhydrin hydrate
-

- $0.00 / 25KG
- 2023-09-12
- CAS:485-47-2
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month