Pyridoxal phosphate: a coenzyme in many enzymatic processes
Nov 10,2023
Pyridoxal phosphate (Pyridoxal 5′-Phosphate, P5P, PLP) is one of the active forms of vitamin B6, which is produced by pyridoxal kinase-mediated reactions. It functions as a coenzyme in many enzymatic processes, including decarboxylation, deamination, transamination, racemization, and others. Enzymes, requiring pyridoxal phosphate, are commonly termed PLP-dependent enzymes, and they are widely involved in crucial cellular metabolic pathways in most of (if not all) living organisms.

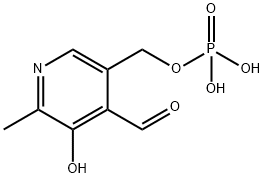
Structure of pyridoxal phosphate
Pyridoxal phosphate has one heteroaromatic pyridine ring, one aldehyde group, a hydroxyl group, and a phosphate group.
The aldehyde group of pyridoxal phosphate makes it possible to form imine with free amino group (e.g., the internal aldimine, formed between pyridoxal phosphate and the conserved lysine residue and external aldimine formed between pyridoxal phosphate and substrate amino group). The heteroaromatic pyridine ring of pyridoxal phosphate enables it to stabilize carbanionic intermediate formed for most (if not all) of the PLP-dependent enzymes, except aminomutase family (radical-initiated reaction).The hydroxyl group could function as a proton donor or acceptor. The role of 5′-phosphate group was less mentioned or elaborated. It was proposed that the phosphate group of pyridoxal phosphate played a role as a general acid/base for accepting or donating a proton in the reaction catalyzed by glycogen phosphorylase.
Mechanism of catalytic reaction
PLP-dependent enzymes catalyze a wide variety of reaction types and usually have a conserved lysine residue in the active site for pyridoxal phosphate binding. The ε-amino group of the lysine residue and the aldehyde group of pyridoxal phosphate forms a Schiff-base structure. Because this Schiff-base structure is linked through a protein-associated lysine residue, it is commonly referred as internal aldimine. After substrate (amino acid or amine) binding, the internal aldimine breaks up and a new Schiff base structure is formed between the amino group of substrate and aldehyde group of pyridoxal phosphate via a gem-diamine intermediate.This newly formed Schiff base is generally termed external aldimine to distinguish it from Schiff-base structure linked with the lysine residue in proteins. The aldimine exchange has been termed transaldimination. This external aldimine formation is common in many PLP-containing enzymes, but once external aldimine is formed, the subsequent reaction mediated by any given enzyme differs, which is dictated primarily by active site conformation or more specifically by the biochemical characteristics of the active site residues that interact with the specific chemical groups of the external aldimine.
Aminotransferase catalyzes the reversible transformation between an amino acid and α-keto acid. After external aldimine formation, deprotonation at Cα leads to a carbanionic intermediate or quinonoid intermediate. Reprotonation at C4′ position of pyridoxal phosphate leads to a ketimine intermediate and one H2O molecule is added to the intermediate Cα. The half-reaction proceeds to have α-keto acid involved to regenerate pyridoxal phosphate and another amino acid. This is a commonly accepted reaction mechanism. In the process, proton transfer between Cα and C4′ was suggested to be promoted by the conserved Lys residue, which is involved in the formation of internal aldimine.
In PLP-dependent decarboxylase, pyridoxal phosphate serves as an electron sink to delocalize the unbounded electrons and to orientate enzyme-substrate in a specific orientation, both of which contribute to the decarboxylation and formation of a quinonoid intermediate. The protonation at Cα leads to an imine formation, which is attacked by Lys amino group to lead to one Schiff base formation between Lys residue and the pyridoxal phosphate (internal aldimine). At the same time, the amine product is released. This is the generally accepted mechanism for the decarboxylation process.
References
[1] Current Advances on Structure-Function Relationships of Pyridoxal 5′-Phosphate-Dependent Enzymes. doi:10.3389/fmolb.2019.00004
- Related articles
- Related Qustion
- Biosynthesis of Pyridoxal Phosphate: A Crucial Coenzyme in Biochemical Reactions Jan 24, 2024
Pyridoxal phosphate is a crucial coenzyme in biochemical reactions. It is synthesized through DXP-dependent and R5P-dependent pathways, with implications for therapeutics and health.
- Role of Pyridoxal phosphate in neonatal epileptic encephalopathy Nov 20, 2023
PLP may be effective in treating neonatal epileptic encephalopathy (NEE).
N,N,N',N'-Tetramethyldiaminomethane is a versatile compound with valuable properties in organic synthesis, but its flammability and toxicity require careful handling.....
Nov 10,2023APINo, apalutamide will not cure your prostate cancer, but it can help control it by acting to delay the need for chemotherapy.....
Nov 10,2023DrugsPyridoxal phosphate
54-47-7You may like
Pyridoxal phosphate manufacturers
- Pyridoxal phosphate
-

- $0.00 / 1kg
- 2024-04-29
- CAS:54-47-7
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 2000
- Pyridoxal phosphate
-

- $180.00 / 1Kg/Bag
- 2024-04-22
- CAS:54-47-7
- Min. Order: 1Kg/Bag
- Purity: 98.5% up / EP
- Supply Ability: 20 tons
- Pyridoxal phosphate
-

- $0.00 / 1kg
- 2024-04-22
- CAS:54-47-7
- Min. Order: 1kg
- Purity: 99.8%
- Supply Ability: 1000 kg