Synthesis and Research Applications of 3-Chloropyrazine-2-carbonitrile in Drug Discovery
Feb 22,2023
Gneral Description
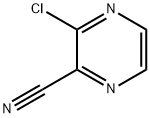
3-Chloropyrazine-2-carbonitrile is a heterocyclic compound with the CAS number 55557-52-3. It is a colorless solid that is used in some organic synthesis, and it has been studied for its potential applications in scientific research. 3-Chloropyrazine-2-carbonitrileis a derivative of pyrazine, which is a six-membered aromatic heterocycle with two nitrogen atoms. This compound has been studied for its potential applications in scientific research, such as in the synthesis of drugs, in the development of new catalysts, and in the synthesis of polymers.

Figure 1. Properties of 3-Chloropyrazine-2-carbonitrile
Synthesis
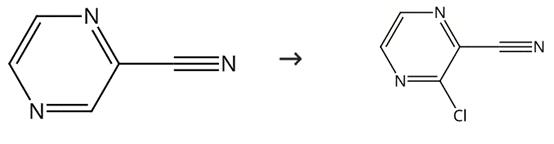
Figure 2. Synthesis of 3-chloropyrazine-2-carbonitrile
Add sulfuryl chloride (21.2 mL, 260.8 mmol) to a solution of pyrazine-2-carbonitrile (6.90 g, 65.65 mmol) in toluene (48 mL) and DMF (5 mL) over 10 min. Stir the reaction mixture for 30 min in an ice bath. Allow the reaction mixture to warm up gradually to room temperature. Stir the resulting mixture for 5 h. Decant the toluene layer. Extract the reddish oil residue three times with diethyl ether. Quench the combined toluene and ether layers with ice water. Cool the resulting mixture in an ice bath. Neutralize the combined layers with solid NaHCO3 and separate. Extract the aqueous layer with diethyl ether. Wash the combined organic layers with water. Dry the resulting mixture over anhydrous Na2SO4 and filter. Evaporate the solvent under reduced pressure to afford the crude title compound. Purify the crude product by silica gel chromatography (eluent: 100% dichloromethane) to obtain 3- chloropyrazine-2-carbonitrile.1
Research Applications
Intermediate in synthesis of cathepsin C inhibitors
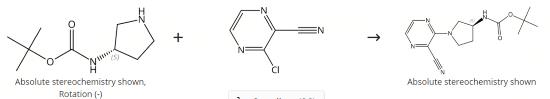
Figure 2. Intermediate in synthesis of cathepsin C inhibitors
To a solution of 1,1-dimethylethyl (3S)-3-pyrrolidinylcarbamate (667 mg, 3.58 mmol) and 3-Chloropyrazine-2-carbonitrile (500 mg, 3.58 mmol) in THF (5.0 mL) was added Et3N (1.00 mL, 7.17 mmol). The reaction vial was capped and heated to 80 °C for 15 h. Upon cooling, the reaction mixture was diluted with EtOAc (~20 mL) and washed with brine (~30 mL). The aqueous layer was extracted with EtOAc (~ 20 mL). The combined organic layers were dried over Na2SO4, filtered, and concentrated in vacuo to afford the title compound (1.07 g, >100%, contained some residual solvent) as a tan solid.2
Intermediate in synthesis of substituted pyrazino[2',3':4,5]thieno[3,2-d]pyrimidines
![Intermediate in synthesis of substituted pyrazino[2',3':4,5]thieno[3,2-d]pyrimidines.png Intermediate in synthesis of substituted pyrazino[2',3':4,5]thieno[3,2-d]pyrimidines.png](/NewsImg/2023-02-15/6381208733012020418021473.jpg)
Figure 3. Intermediate in synthesis of substituted pyrazino[2',3':4,5]thieno[3,2-d]pyrimidines
To a soln of 3-Chloropyrazine-2-carbonitrile (3.82 g, 15.64 mmol) in EtOH (100 mL) was added NaSH (20 mmol) and the mixture was stirred at r.t. for 3 h (TLC monitoring). EtOH was evaporated under reduced pressure and the residue was dissolved in H2O and 2 M HCl was carefully added to neutralize the suspension. After evaporation of the solvent, the residue was dissolved in acetone (40 mL) and ClCH2CN (1 mL, 15.64 mmol), K2CO3 (2.16 g, 15.64 mmol) and a catalytic amount of KI were added. The resulting mixture was stirred at r.t. for 1 h and then the resulting residue was separated by filtration and washed with acetone (3 × 15 mL). The filtrate was evaporated under reduced pressure, the resulting residue was dissolved in THF (200 mL), piperidine (1.55 mL, 15.64 mL) was added and the mixture was stirred at reflux temperature for 5 h. After cooling, the soln was concentrated to dryness and the residual material was purified by column chromatography (THF-hexanes, 1:1) to give 7-Aminothieno[2,3-b]pyrazine-6-carbonitrile. 2.31 g, 84%. mp 205-206 °C.3
Advantages and Limitations for Lab Experiments
The advantages of using 3-Chloropyrazine-2-carbonitrile in lab experiments include its relatively simple synthesis, its high purity, and its low cost. Furthermore, 3-Chloropyrazine-2-carbonitrile is a stable compound and is not easily degraded by light or heat. However, there are some limitations to using 3-Chloropyrazine-2-carbonitrile in lab experiments. For example, its solubility in water is relatively low, and it is not soluble in many organic solvents. In addition, 3-Chloropyrazine-2-carbonitrile is not very soluble in aqueous solutions, which can make it difficult to work with in some experiments.
Reference
1. Loidreau, Y., Marchand, P., Dubouilh-Benard, C., Nourrisson, M. R., Duflos, M., Lozach, O., ... & Besson, T. (2012). Synthesis and biological evaluation of N-arylbenzo [b] thieno [3, 2-d] pyrimidin-4-amines and their pyrido and pyrazino analogues as Ser/Thr kinase inhibitors. European journal of medicinal chemistry, 58, 171-183.
2. World Intellectual Property Organization, WO2011029633 A1 2011-03-17.
3. Fernandez-Mato, A., Peinador, C., & Quintela, J. M. (2011). Synthesis and Characterization of Substituted Pyrazino [2′, 3′: 4, 5] thieno [3, 2-d] pyrimidines and Related Molecules. Synthesis, 2011(06), 943-953.
- Related articles
- Related Qustion
- Unveiling the Potential of 3-Chloropyrazine-2-carbonitrile in Modern Chemistry Apr 22, 2024
3-Chloropyrazine-2-carbonitrile has become a beacon in the field of chemical research due to its unique properties.
Nemorubicin is a member of the structural group of morpholinyl anthracyclines whose lead compounds were originally obtained by Takahashi et al.....
Feb 21,2023API2-Naphthol is produced by sulfonation of naphthalene to 2-naphthalene sulfonic acid, and reaction with caustic soda solution to obtain the sodium salt. Reaction of the sodium salt with sodium hydroxide and treating the melt with sulfuric....
Feb 22,2023Organic Chemistry3-Chloropyrazine-2-carbonitrile
55557-52-3You may like
3-Chloropyrazine-2-carbonitrile manufacturers
- 3-Chloropyrazine-2-carbonitrile
-
- $5.00 / 1KG
- 2024-04-09
- CAS:55557-52-3
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: g-kg-tons, free sample is available
- 3-Chloropyrazine-2-carbonitrile
-

- $0.00/ kg
- 2023-12-24
- CAS:55557-52-3
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 1 ton
- 3-Chloropyrazine-2-carbonitrile
-
- $0.00 / 1KG
- 2022-09-30
- CAS:55557-52-3
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 1ton