The application of Omeprazole
Oct 31,2022
General description
Omeprazole and esomeprazole are proton pump inhibitors (PPIs) and potent inhibitor of gastric acidity which are widely used in the therapy of gastroesophageal reflux and peptic ulcer disease. Omeprazole and esomeprazole therapy are both associated with a low rate of transient and asymptomatic serum aminotransferase elevations and are rare causes of clinically apparent liver injury. Originally approved by the FDA in 1989, omeprazole is a proton-pump inhibitor, used to treat gastric acid-related disorders. These disorders may include gastroesophageal reflux disease (GERD), peptic ulcer disease, and other diseases characterized by the oversecretion of gastric acid. This drug was the first clinical useful drug in its class, and its approval was followed by the formulation of many other proton pump inhibitor drugs. Omeprazole is generally effective and well-tolerated, promoting its popular use in children and adults. 6-methoxy-2-[(4-methoxy-3,5-dimethyl-2-pyridinyl)methylsulfinyl]-1H-benzimidazole is a member of benzimidazoles and a sulfoxide.

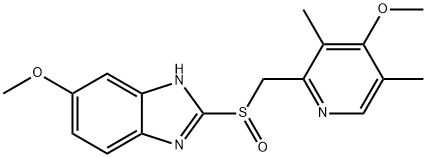
Figure 1 the chemical structure of Omeprazole
Application and Pharmacology
1.All the studied drugs reduced DPPH, but to different extents. However, omeprazole and esomeprazole showed the highest ability to scavenge free radicals (50% ) inhibitory concentrations [IC50s] of the percentage for free radical scavenging activity are 18.7 ± 5.7 and 18.7 ± 5.7, respectively, and the AA equivalents are 83,772 ± 11,887 and 81,732 ± 8,523 mg AA/100 g, respectively). Conversely, lansoprazole, pantoprazole, and rabeprazole might be having no role in this story (IC50s of the percentage for free radical scavenging activity are 49.3 ± 3.1, 49 ± 9.4, and 40.7 ± 7.2, respectively, and the AA equivalents are 30,458 ± 3,884, 32,222 ± 10,377, and 37,876 ± 8,816 mg AA/100 g, respectively). Conclusion: Thus, omeprazole and esomeprazole may confer a significant dual action in gastrointestinal protection by providing potent antioxidant properties in addition to their major role as acid-suppression agents. However, further studies are essential to elucidate the mechanism behind the difference between the drugs of the same class.[1]
2.Evaluate the effectiveness and tolerability of esomeprazole and omeprazole in patients with gastroesophageal reflux disease (GERD). Electronic searches on PubMed, EMBASE, the Cochrane Library, and Clinical Trials. gov databases were carried out for reports up to February 28, 2015. Ten eligible studies from 8 articles were found that enrolled a total of 10,286 patients for meta-analysis. These results revealed a significant difference between esomeprazole vs. omeprazole (RR = 1.06, 95% CI [1.01, 1.10], I2 = 72%, p = 0.01) by subgroup according to dosage by random effects model, and a significant difference between esomeprazole 40 mg vs. omeprazole 20 mg (RR = 1.07, 95% CI [1.004, 1.14], I2 = 78%, p = 0.04) based on healing rate as determined by endoscopy, using a random effects model. A significant difference between esomeprazole 20 mg and omeprazole 40 mg (RR = 0.68, 95% CI [0.47, 0.97], I2 = not applicable, p = 0.03) was also found in comparing relief of symptoms by random effects model. There were no significant differences in outcomes between other subgroups, including tolerability. Based on these results, a high dose of esomeprazole is recommended for GERD treatment and control in adults.[2]
Synthesis
The title method for preparing esomeprazole sodium includes allowing sodium hydroxide and omeprazole to react to obtain first intermediate, reacting in cyclohexane and purified water to obtain hydrate, and then reacting with auxiliary resolution reagents (D-diethyl tartrate, tetraisopropyl titanate and triethylamine) and main resolution reagent S-mandelic acid to obtain second intermediate; reacting with dichloromethane and sodium sulfite, filtering, layering, and extracting organic layer with dichloromethane to obtain third intermediate; and dissolving in Et acetate under stirring to appearance of white turbidity, filtering, dropwise adding sodium methylate under stirring to sep. out large amount of white solid, filtering, washing filter cake with Et acetate, and drying to obtain the final product. By controlling addition amounts of specific reaction solvent and water and controlling specific reaction temperature, time and pH value, the invention can obtain product with purity higher than 99% and yield higher than 32%.
Reference
1.Qi Q., Wang R. & Liu L. et al., "Comparative effectiveness and tolerability of esomeprazole and omeprazole in gastro-esophageal reflux disease: A systematic review and meta-analysis," Int. Journal of Clinical Pharmacology and Therapeutics, Vol.53, No.10(2015), pp.803-810.
2.Abed M. N., Alassaf F. A. & Jasim M. H. M. et al., "Comparison of Antioxidant Effects of the Proton Pump-Inhibiting Drugs Omeprazole, Esomeprazole, Lansoprazole, Pantoprazole, and Rabeprazole," Pharmacology, Vol.105, No.11-12(2020), pp.645-651.
- Related articles
- Related Qustion
Methacrylic acid appears as a clear colorless liquid (or low-melting solid) with a pungent odor. Corrosive to metals and tissue. Flash point 170°F. Melting point 61°F.....
Oct 31,2022Organic Synthesis IntermediateDiethylamino hydroxybenzoyl hexyl benzoate is a light-stabilized UV-A absorber.....
Nov 1,2022SupplementsOmeprazole
73590-58-6You may like
- Omeprazole
-

- $0.00 / 1kg
- 2024-04-29
- CAS:73590-58-6
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 500
- Omeprazole
-

- $0.00 / 1kg
- 2024-04-29
- CAS:73590-58-6
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 1tons
- Omeprazole
-

- $10.00/ kg
- 2024-04-28
- CAS:73590-58-6
- Min. Order: 1kg
- Purity: 99.8%
- Supply Ability: 10000ton