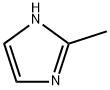
The different applications of 2-methylimidazole
Jul 29,2022
Introduction
2-Methylimidazole is an organic compound with the molecular formula C4H6N2. 2-methylimidazole, also known as dimethyl imidazole, is white acicular crystal or crystalline powder at room temperature[1]. Relative molecular weight 82.11. Melting point 145 ~ 146 ℃. Boiling point 267 ℃. Can sublimate. Flash point 160 ℃. Insoluble in ether and cold benzene, easily soluble in water, alcohols and ketones. Irritating to skin and mucous membranes. Oral ld501400mg / kg in mice. 2-Methylimidazole is an intermediate for the production of anti trichomonad pesticide metronidazole. It is also the curing agent and curing accelerator of epoxy resin and other resins. It occupies a special position in the amine curing agent. When it is used as the medium temperature curing agent of epoxy resin, it can be used alone. After heat treatment in a short time, the cured product with high thermal deformation temperature can be prepared. But it is mainly used as curing accelerator for powder forming and powder coating. It can be obtained by dehydrogenation of 2-methylimidazoline[1].

Picture 1 The physical map of 2-methylimidazole
Structure
The crystal structure of the title compound, C4H6N2, has been determined using X-ray diffraction at 100 K. The crystal of 2-methylimidazole is in orthorhombic crystal system with space group P212121 (Z = 4), lattice parameters: a = 5.9957(12) Å, b = 8.1574(16) Å and c = 9.7010(19) Å, V = 474.47(16) Å. The molecule of 2-methylimidazole is approximately planar. The maximum deviation from the least-squares imidazole plane, calculated for all non-H atoms is 0.006(2) Å. N–H···N hydrogen bonds link the molecules together, forming infinite chains of hydrogen bond pattern C(4) defined by the graph-set analysis. Two chains, which are almost antiparallel to each other, pass through each unit cell. The dihedral angle between the mean planes of molecules forming these intersecting chains is 76.90(4)°. No evidence was found for disorder of the hydrogen-bonding proton between the atoms N1 and N3. The significance of this study lies in the analysis of the interactions occurring via hydrogen bonds in this structure, as well as, in the comparison drawn between the molecular structure of 2-methylimidazole and those of several of other imidazole derivatives possessing a hydrogen atom in the N1 position[2].
The application of 2-methylimidazole in the food products industry
The use of chemical preservative compounds is common in the food products industry. Caramel color is the most usual additive used in beverages, desserts, and breads worldwide[4]. During its fabrication process, 2- and 4-methylimidazole (MeI), highly carcinogenic compounds, are generated. In these cases, the development of reliable analytical methods for the monitoring of undesirable compounds is necessary. The primary procedure for the analysis of 2- and 4-MeI is using LC- or GC-MS techniques. These procedures are time-consuming and require large amounts of organic solvents and several pretreatment steps. This prevents the routine use of this procedure. This paper describes a rapid, efficient, and simple method using capillary electrophoresis (CE) for the separation and determination of 2- and 4-MeI in caramel colors. The analyses were performed using a 75 μm i.d. uncoated fused-silica capillary with an effective length of 40 cm and a running electrolyte consisting of 160 mmol L–1 phosphate plus 30% acetonitrile. The pH was adjusted to 2.5 with triethylamine. The analytes were separated within 6 min at a voltage of 20 kV. Method validation revealed good repeatability of both migration time (<0.8% RSD) and peak area (<2% RSD). Analytical curves for 2- and 4-MeI were linear in the 0.4–40 mg L–1 concentration interval. Detection limits were 0.16 mg L–1 for 4-MeI and 0.22 mg L–1 for 2-MeI. The extraction recoveries were satisfactory. The developed method showed many advantages when compared to the previously used method.
The application of 2-methylimidazole in the determination of acids
Anhydrides of dicarboxylic acids are commonly determined using titrimetric methods for the determination of acids that are formed on the interaction of anhydride with primary and secondary amines[5]. The aniline method allows only the potentiometric titration of the resulting compound because of the low basicity of the amine. None of the methods for the determination of maleic anhydride is selective for other anhydrides of dicarboxylic acids. 2-methylimidazole is a compound that, depending on the ambient conditions, can exhibit either basic or acidic properties because of the presence of the pyrrolic and pyridinic nitrogen atoms in their structure. The pyrrolic nitrogen atoms exhibit properties of secondary amines and can react with anhydrides of dicarboxylic acids, yielding corresponding amides. The pyridinic nitrogen atom is a nucleophile and can catalyze reactions of epoxy groups and anhydrides of dicarboxylic acids with hydroxyl-containing compounds. The selection of 2-methylimidazole as a reagent for the photometric determination of maleic anhydride in the dimethyl sulfoxide medium was very usefully.
Reference
1 Hachua, Barbara, Maria Nowak, and Joachim Kusz. "Crystal and molecular structure analysis of 2-methylimidazole." Journal of chemical crystallography 40.3 (2010): 201-206.
2 Hachu?a B, Nowak M, Kusz J. Crystal and molecular structure analysis of 2-methylimidazole[J]. Journal of chemical crystallography, 2010, 40(3): 201-206.
3 Yen S K, Su M, Wang K Y, et al. Study of draw solutes using 2-methylimidazole-based compounds in forward osmosis[J]. Journal of Membrane Science, 2010, 364(1-2): 242-252.
4 Petruci J F S, Pereira E A, Cardoso A A. Determination of 2-methylimidazole and 4-methylimidazole in caramel colors by capillary electrophoresis[J]. Journal of agricultural and food chemistry, 2013, 61(9): 2263-2267.
5 Yu. M. Evtushenko; B. E. Zaitsev; V. M. Ivanov; K. M. Gitis (2000). 2-methylimidazole as a reagent for the photometric determination of maleic anhydride. , 55(2), 142–145.
- Related articles
- Related Qustion
D-panthenol is readily converted to pantothenic acid which is widely distributed into body tissues, mainly as coenzyme A, which is necessary for important reactions in material metabolism.....
Jul 29,2022APIL-Hydroxyproline has a special flavor and can be used as a fragrance raw material.....
Jul 29,2022API2-Methylimidazole
693-98-1You may like
2-Methylimidazole manufacturers
- 2-Methylimidazole
-

- $6.00 / 1KG
- 2024-04-30
- CAS:693-98-1
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20TONS
- 2-Methylimidazole
-

- $6.00 / 1KG
- 2024-04-19
- CAS:693-98-1
- Min. Order: 1KG
- Purity: More than 99%
- Supply Ability: 2000KG/MONTH
- 2-Methylimidazole
-

- $10.00 / 1KG
- 2024-04-10
- CAS:693-98-1
- Min. Order: 1KG
- Purity: 99.9%
- Supply Ability: 1000Ton/month